Method for conducting catalytic hydrogenation on nitrogen-containing unsaturated heterocyclic compound
A heterocyclic compound and unsaturated technology, which is applied in the field of catalytic hydrogenation, can solve the problems that the catalytic effect needs to be improved, and the scope of application of the catalyst substrate is limited.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
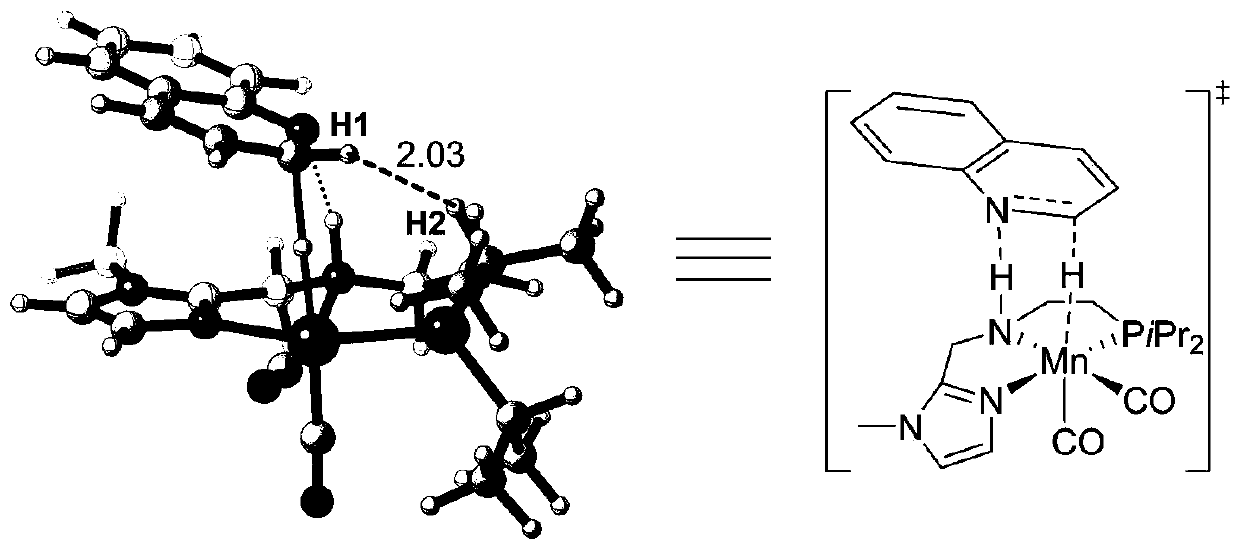
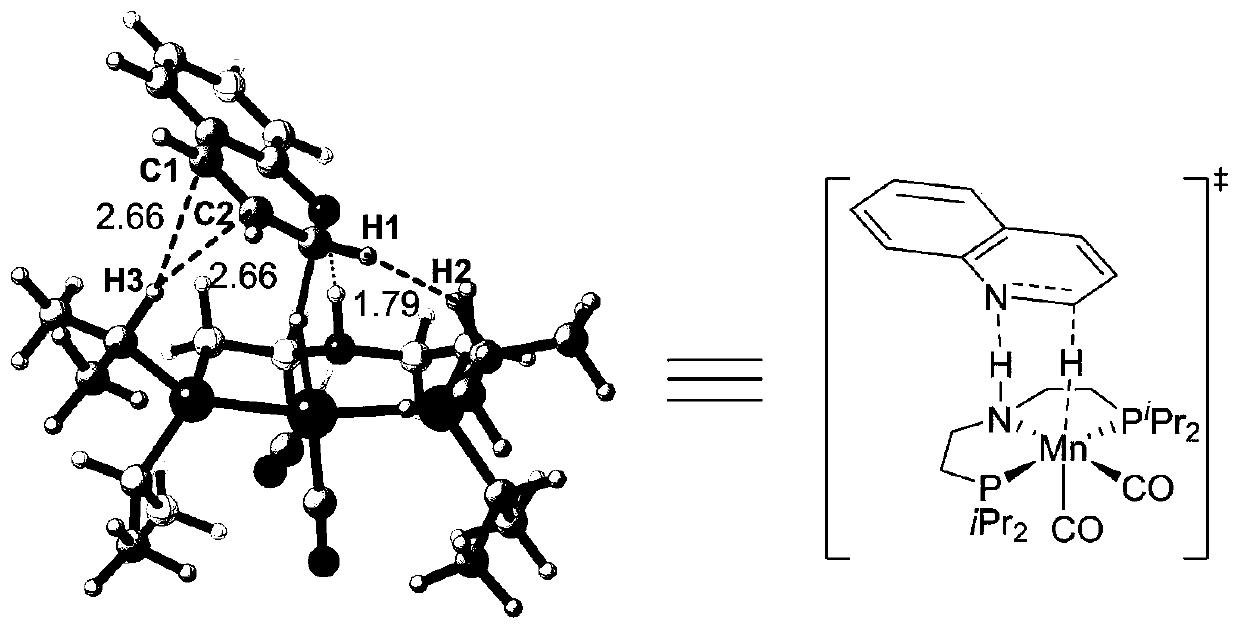
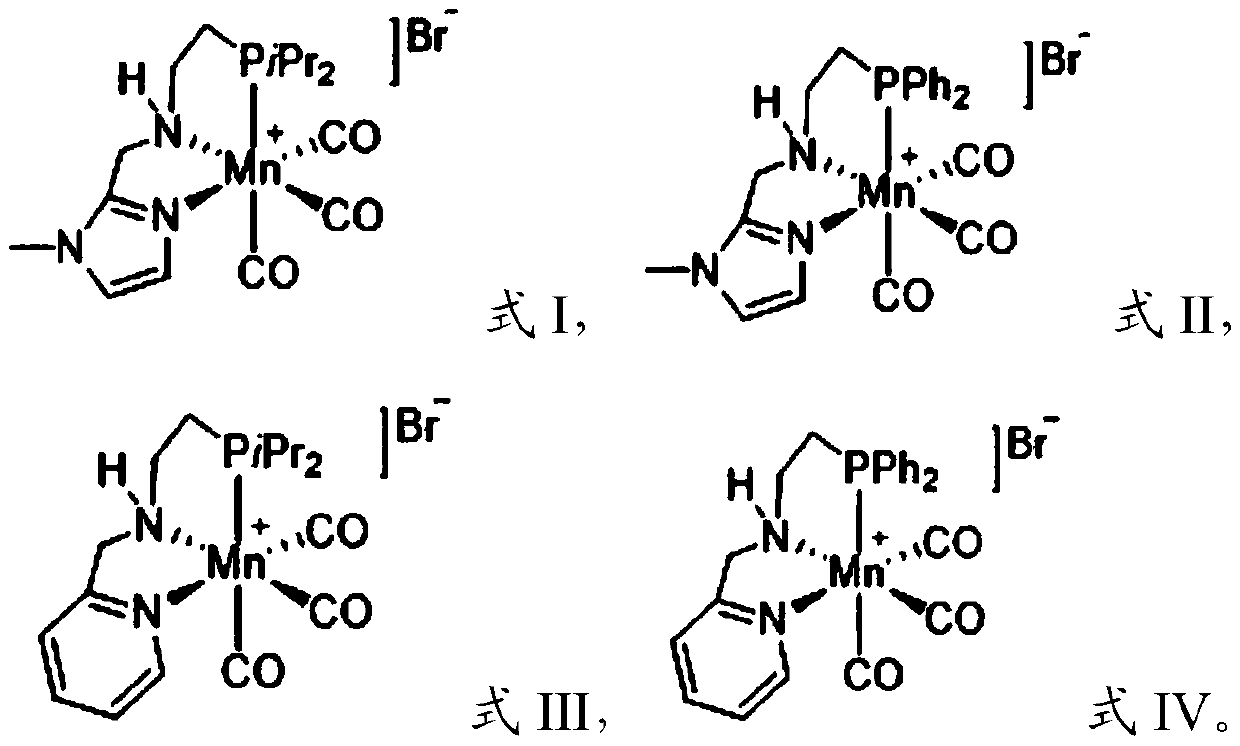
[0047]The manganese catalyst that the present invention adopts is NNP type pincer manganese catalyst, and it has cheap, easy to get, the advantage of low toxicity with respect to noble metal catalyst; With respect to existing cheap metal iron catalyst or cobalt catalyst, has bottom It has the advantages of wide material applicability and high yield of target products; compared with PNP-type pincer manganese catalyst, it has stronger electron donating ability and smaller steric hindrance, so it shows higher reactivity in a series of hydrogenation reactions , the target product yield can reach up to 99%. In the present invention, the manganese catalysts [Mn]-1, [Mn]-3 and [Mn]-4 are preferably synthesized by reference method (Chem.Sci.2017,8,3576; J.Am.Chem.Soc. 2017,139,11941; Angew.Chem.Int.Ed.2018,57,13439), the preparation method of the manganese catalyst [Mn]-2 preferably comprises the following steps:
[0048] Mix 2-chloroethylamine hydrochloride, trimethylchlorosilane, t...
Embodiment 1
[0072] Preparation has the manganese catalyst of structure shown in formula II, i.e. manganese catalyst [Mn]-2, comprises the following steps:
[0073] (1) Synthesis of 2-chloro-N,N-bis(trimethylsilyl)ethylamine
[0074]
[0075] Add 2-chloroethylamine hydrochloride (4.6g, 40mmol), triethylamine (NEt 3 , 18mL, 132mmol) and dichloromethane 50mL, a dichloromethane solution (20mL) of trimethylchlorosilane (TMSCl, 90mmol, 9.8g, 11.4mL) was added to the resulting system, and the reaction was stirred at room temperature for 12h; the reaction ended Afterwards, excess triethylamine, trimethylchlorosilane and dichloromethane were removed under reduced pressure, 60 mL of n-hexane was added to the resulting residue, stirred at room temperature for 30 min, and NEt was removed by filtration. 3 HCl, use a rotary evaporator to carry out rotary evaporation to the filtrate to remove n-hexane, and then use an oil pump to carry out vacuum distillation, and the obtained fraction is the target...
Embodiment 2
[0095] In a glove box filled with argon, potassium tert-butoxide (5.6mg, 0.05mmol) and manganese catalyst (0.005mmol) were successively added to a 4mL glass bottle with a stirrer, and the manganese catalysts were represented by formulas I to IV The manganese catalyst of the shown structure is respectively denoted as manganese catalyst [Mn]-1, [Mn]-2, [Mn]-3, [Mn]-4), tetrahydrofuran (0.5mL) and quinoline (0.25mmol), Cover the bottle cap, insert a needle with a vent hole on the bottle cap (the length is 3cm, and the aperture of the vent hole is 1mm), the glass bottle is put into the autoclave, and then the autoclave is taken out from the glove box, Replace the argon (3×10bar) in the autoclave with hydrogen, then fill it with 80bar hydrogen, and react at 120°C for 16h; GC quantified and separated by column chromatography to obtain the target product.
[0096] Taking the manganese catalyst [Mn]-1 as an example, the reaction process is as follows:
[0097]
[0098] The struct...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com