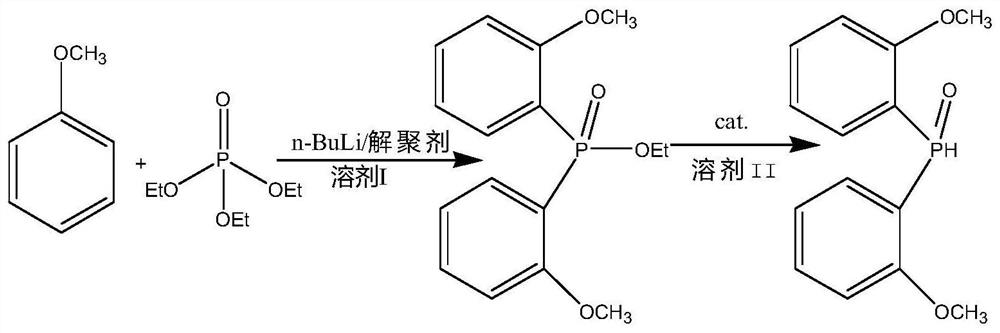
A kind of synthetic method of two (2-methoxyphenyl) phosphine oxides
A technology of methoxyphenyl and phosphine oxides, which is applied in the field of synthesis of bisphosphine oxides, can solve problems such as low yield, long reaction route, and uncontrollable impurities, and achieve high conversion rate, excellent product quality, and reaction simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] The invention provides a kind of synthetic method of bis(2-methoxyphenyl)phosphine oxide, comprising the following steps:
[0030] a) Anisole reacts with triethyl phosphate in a solvent to obtain bis(2-methoxyphenyl) ethyl phosphonate;
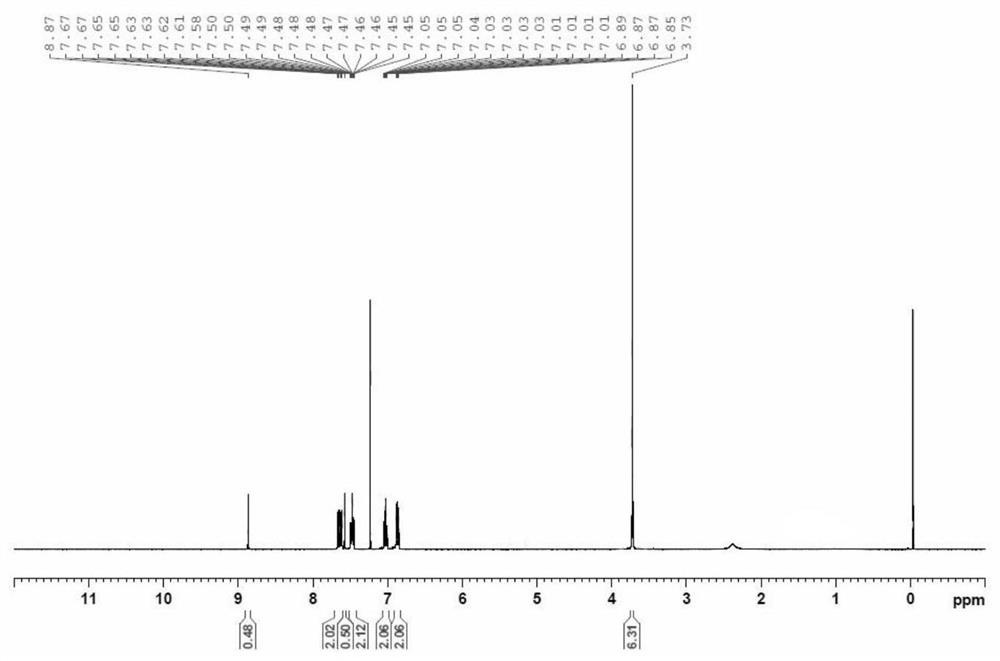
[0031] b) The ethyl bis(2-methoxyphenyl)phosphonate is hydrogenated and reduced in a solvent to obtain bis(2-methoxyphenyl)phosphine oxide.
[0032] In the present invention, first, anisole and triethyl phosphate are reacted in a solvent to synthesize ethyl bis(2-methoxyphenyl)phosphonate. Wherein, the solvent includes but not limited to tetrahydrofuran and / or n-hexane; the reaction is carried out in the presence of a depolymerizing agent and n-butyllithium (n-BuLi) and under anhydrous and oxygen-free conditions; the depolymerizing agent preferably includes Tetramethylethylenediamine and / or hexamethylphosphoric triamide; The mol ratio of described anisole, depolymerization agent, n-butyl lithium and triethyl phosphate is preferably 1: ...
Embodiment 1
[0047] A kind of synthetic method of two (2-methoxyphenyl) phosphine oxides, synthetic route is as follows figure 1 as shown, figure 1 It is a synthetic route diagram of bis(2-methoxyphenyl)phosphine oxide provided by the examples of the present invention. The specific steps are:
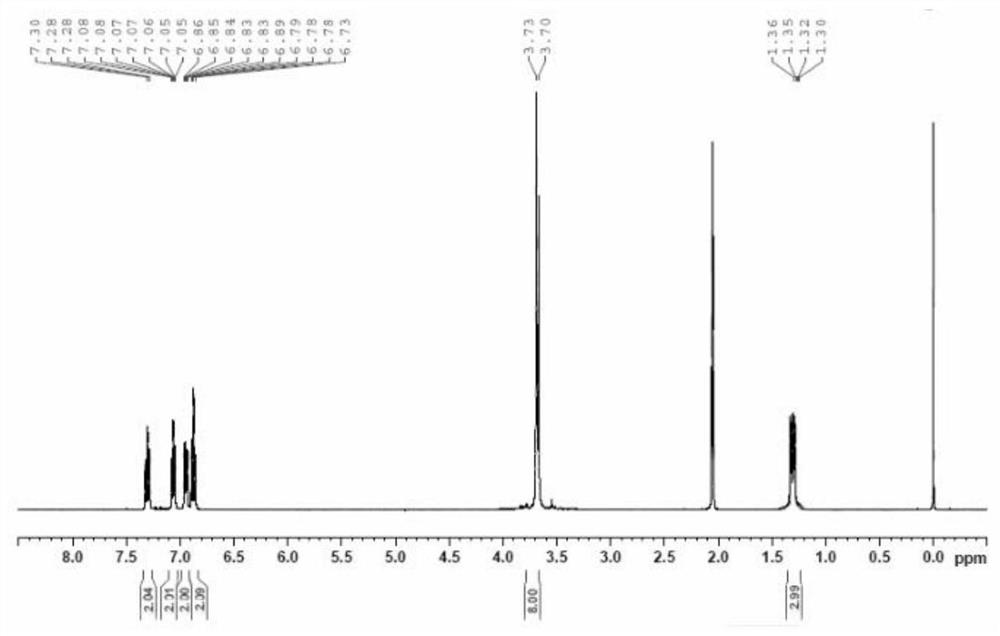
[0048] (1) Preparation of two (2-methoxyphenyl) phosphonic acid ethyl esters
[0049] Under anhydrous and oxygen-free conditions, add 6.48g of anisole and 6.97g of tetramethylethylenediamine into 32.4mL of dry tetrahydrofuran (solvent I), lower the temperature to -30°C, and start adding 39.38mL of 1.6mol / L of n-BuLi solution, after the dropwise addition, stir under temperature control for 1-2 hours, raise the temperature to 20-50°C and stir, track and detect that the reaction is complete, cool down to -30°C, and start adding 5.46g of triethyl phosphate dropwise under temperature control , after the dropwise addition, keep warm for 1 to 2 hours, heat up to 30°C, track and detect the reaction, coo...
Embodiment 2
[0055] A kind of synthetic method of two (2-methoxyphenyl) phosphine oxides, synthetic route is as follows figure 1 As shown, the specific steps are:
[0056] (1) Preparation of two (2-methoxyphenyl) phosphonic acid ethyl esters
[0057] Under anhydrous and oxygen-free conditions, add 6.48g of anisole and 10.45g of tetramethylethylenediamine into 64.8mL of dry n-hexane (solvent I), lower the temperature to -20°C, and start adding 39.38mL of 1.6mol / L of n-BuLi solution, after the dropwise addition is completed, the temperature is controlled and stirred for 1 to 2 hours, and the temperature is raised to 20 to 50°C for stirring. g. After the dropwise addition, keep warm for 1 to 2 hours, heat up to 40°C, track and detect the reaction, cool down to room temperature, slowly drop the reaction solution into ice water containing 10% hydrochloric acid under stirring, separate the liquid, and wash with water , recovered n-hexane under reduced pressure, precipitated a large amount of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com