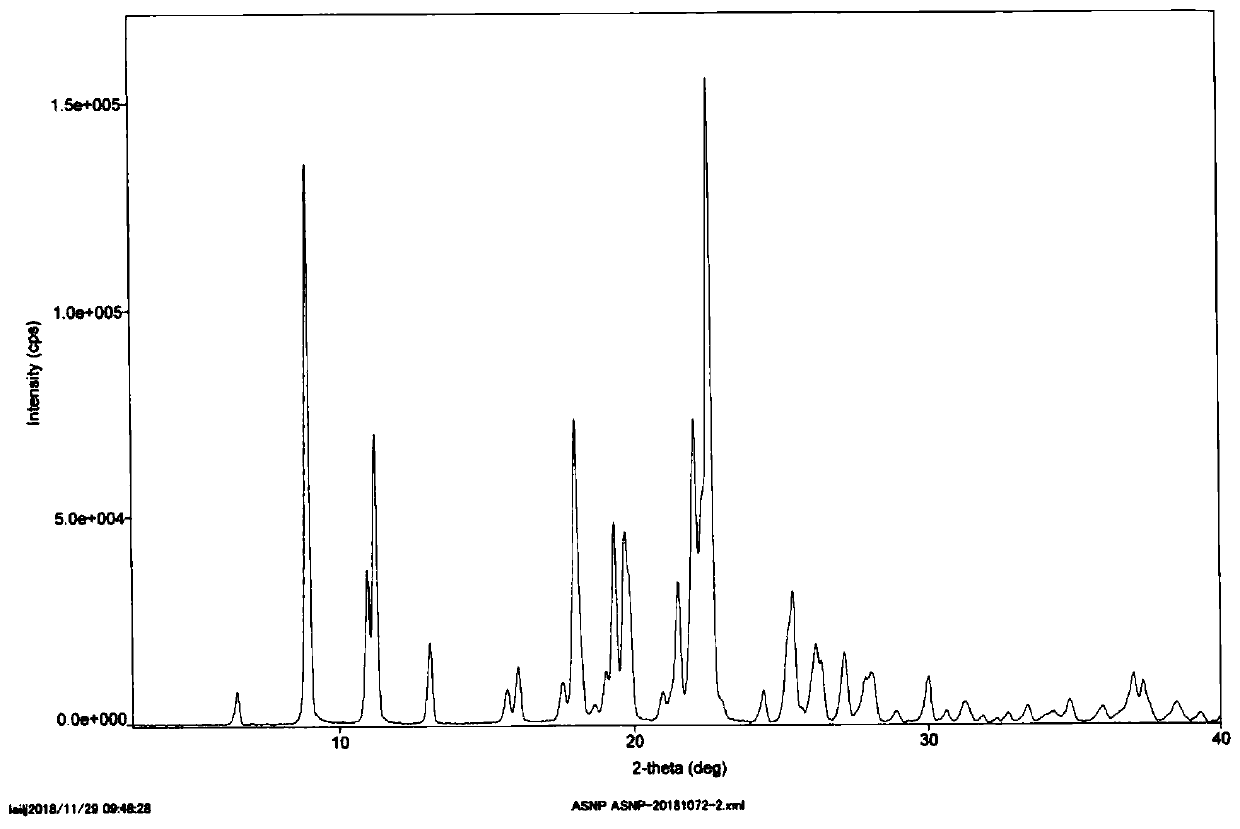
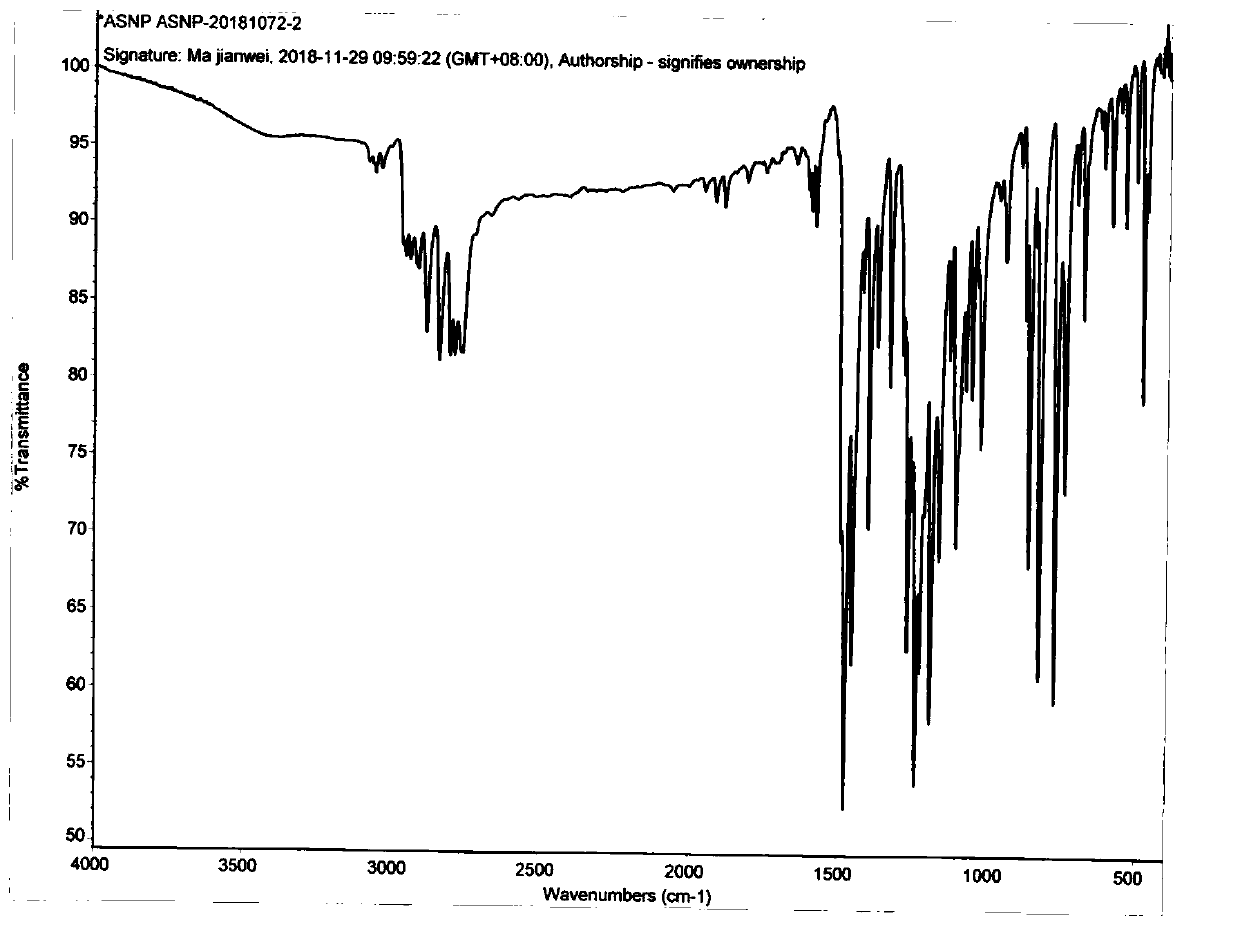
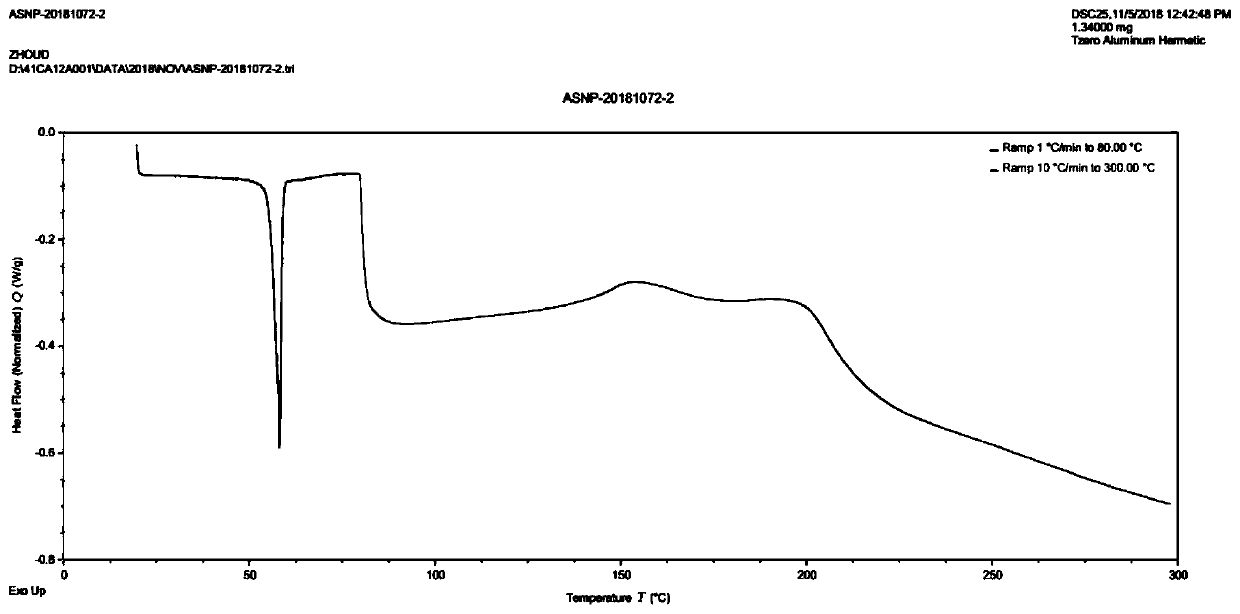
Method for preparing asenapine
An intermediate and reaction technology, which is applied in the field of synthesis of organic compounds, can solve the problems of large environmental pollution, lengthy synthesis routes, and the use of flammable and explosive reagents, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0141]
[0142] Step (b-1): Synthesis of compound VI:
[0143] Add 35.2g of compound V, 63.3g of 48% hydrobromic acid aqueous solution into a 200ml reaction bottle, add 18.4g of sulfuric acid dropwise at room temperature, heat up to 50°C and keep it for 10h, cool to room temperature, add 100ml of dichloromethane, add 50ml of water and 50ml of Wash the organic layer with aqueous sodium carbonate solution, add 10g of sodium sulfate to dry, filter, add 63.1g of triethyl phosphite and 8.6g of anhydrous zinc bromide to the organic layer at one time, heat the reaction solution to 40°C, and stir the reaction mixture After 10 hours, cool slightly, add 100ml of water to wash, separate the water layer, collect the organic phase, and evaporate the solvent to dryness under reduced pressure to obtain compound VI (54.8g, 95.0%).
[0144] Step (b-2): Synthesis of Compound VIII
[0145] Under nitrogen protection, 54.0g of compound VI was dissolved in 500ml of tetrahydrofuran, and 30.1g of...
Embodiment 2
[0167]
[0168] 73.0 g of compound IV was dissolved in 220 g of hypophosphorous acid, cooled to 0-15°C, and 64.0 g of 30% aqueous sodium nitrite solution was added dropwise, and the addition was completed within 2-3 hours. After dropping, control the temperature at 10-20°C and keep stirring for 5-9 hours. Control the temperature below 30°C and add 160g of ammonia water dropwise, adjust the pH of the water layer to 8-9, add 300ml of n-hexane, wash the organic layer with 160ml of water, and evaporate the organic layer to dryness under reduced pressure at 45-50°C to obtain a crude product.
[0169] Add 230ml of n-heptane and 17ml of toluene to the crude product, raise the temperature to 40-50°C and stir to dissolve, then cool and crystallize at a rate of 5-10°C per hour, cool to 20-25°C and stir and crystallize for 1.0-1.5 h, after a large amount of solids are precipitated, cool down to -5~0°C, keep stirring for 1.0h, filter, collect the crystallized asenapine, and dry under v...
Embodiment 3
[0171]
[0172] Step (c-1): Synthesis of Compound Ⅺ
[0173] Add 50.0g of compound V and 75.0g of 48% hydrobromic acid aqueous solution into a 250ml reaction bottle, add 14.0g of sulfuric acid dropwise at room temperature, heat up to 60°C and keep it for 13h, cool to room temperature, add 100ml of toluene, and wash the organic layer with 35.0g of water in turn Wash with 28.0 g of 2.5% aqueous sodium carbonate solution, stand to separate the layers, add 65.2 g of triphenylphosphine to the organic layer, stir at 60° C. for 10 h, then cool to ambient temperature, and filter to obtain compound XI (118.8 g, 95.2%).
[0174] Step (c-2): Synthesis of Compound VIII
[0175]Under nitrogen protection, 102.4g of compound XI was dissolved in 1000ml of tetrahydrofuran, and 37.4g of 5-chlorosalicylaldehyde was added, and 15.8g of pyridine was added dropwise. After the reaction was completed, 600ml of water and 600ml of hexane were added, and saturated with 400ml of sodium carbonate solut...
PUM
| Property | Measurement | Unit |
|---|---|---|
| crystallization temperature | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com