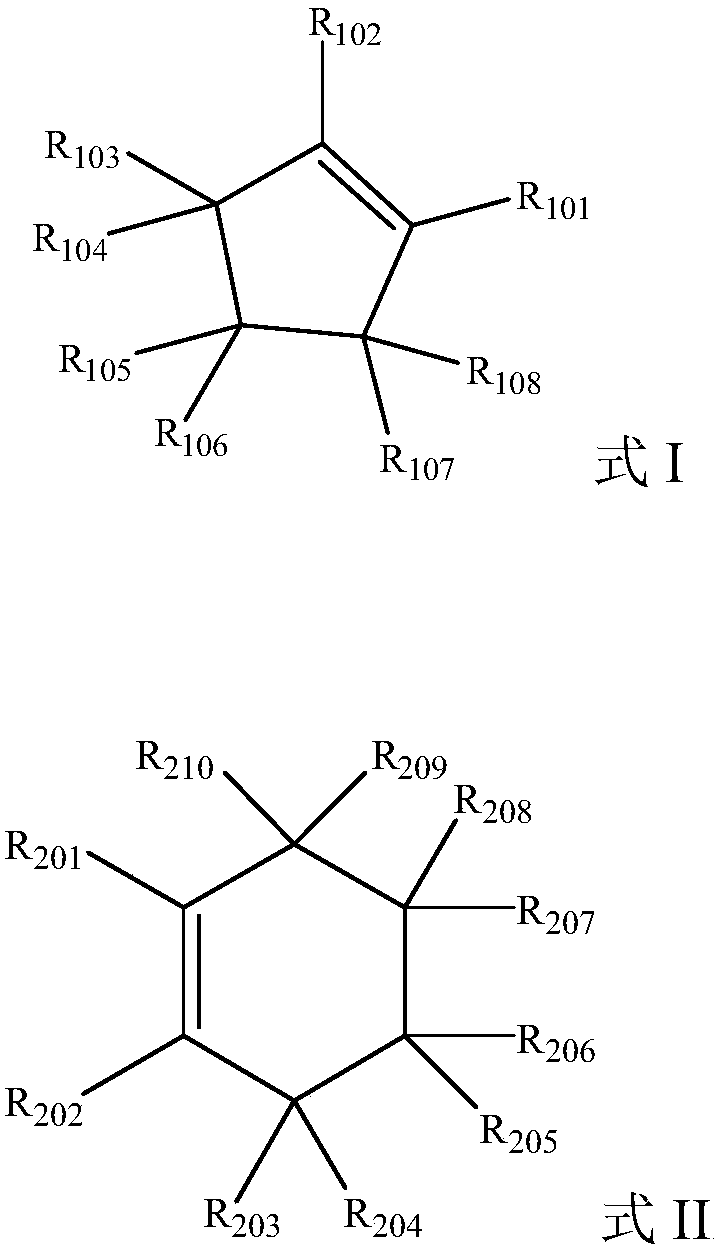
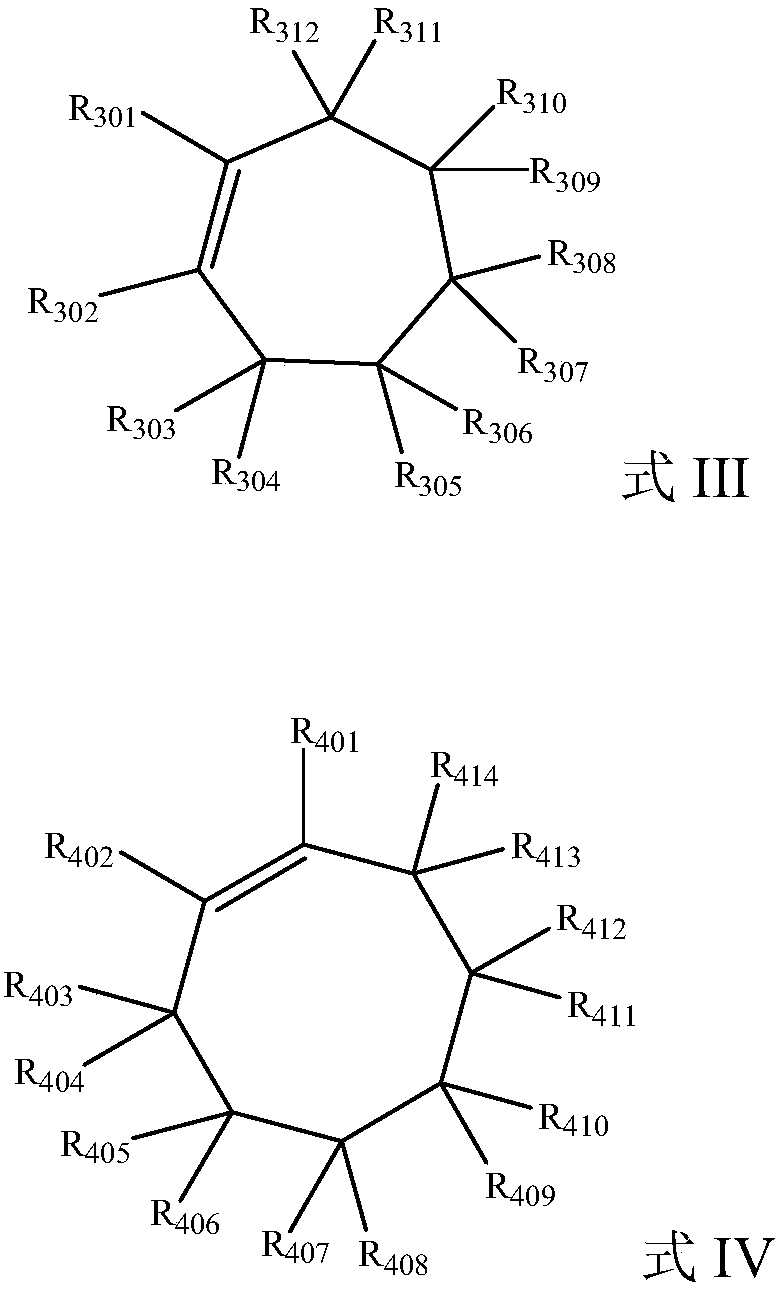
Preparation method of dicarboxylic acid
A technology of dicarboxylic acid and monocarboxylic acid, applied in the preparation of carboxylate, carboxylate, preparation of organic compounds, etc., can solve the problems of slow hydration reaction rate, limited reaction rate, low solubility, etc., and achieve high Reactivity and selectivity, high conversion and selectivity, less corrosive effect of equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
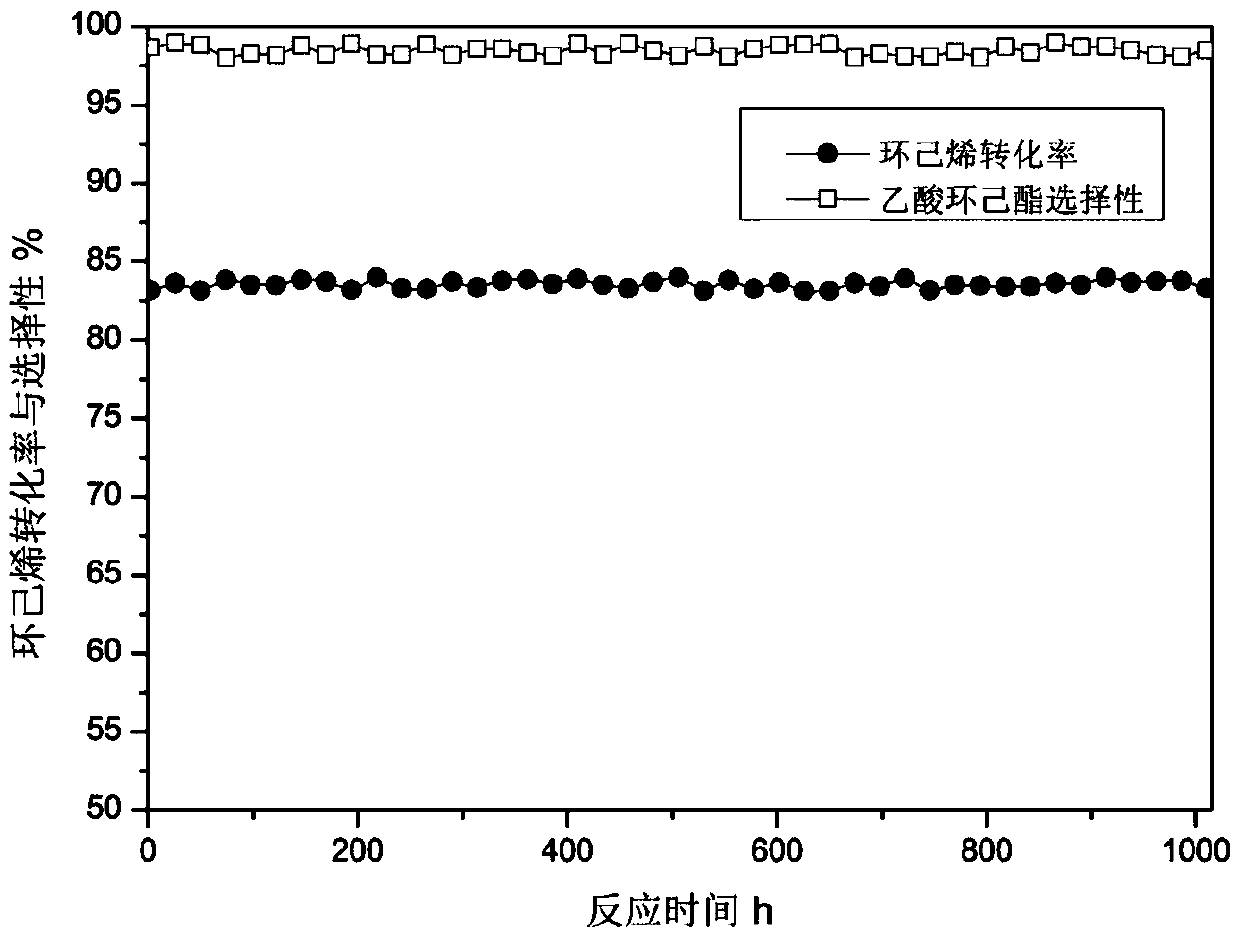
[0069] 60ml of macroporous strongly acidic ion-exchange resin Amberlyst15 is packed into the middle part of the stainless steel tubular fixed-bed reactor, and the top and bottom of the catalyst are filled with quartz sand respectively. The temperature of the reactor was raised to 90° C., and the reaction pressure was 0.1 MPa. The reactant cyclohexene and acetic acid enter the reactor separately, and the molar ratio of acetic acid to cyclohexene is 4:1. The feed space velocity of cyclohexene is 1.0g.g -1. h -1 . The reaction was run continuously for 250 hours. After the reaction product was collected, the composition of the product was analyzed by gas chromatography. The conversion rate of cyclohexene was 85.5%, and the selectivity of cyclohexyl acetate was 98.1%.
[0070] The addition reaction product is rectified to obtain cyclohexyl acetate with a purity greater than 99.5%. The cyclohexyl acetate was further oxidized, and the specific reaction steps were as follows: 14...
Embodiment 2
[0072] 60ml of macroporous strongly acidic ion-exchange resin Amberlyst35 is packed into the middle part of the stainless steel tubular fixed-bed reactor, and the top and bottom of the catalyst are filled with quartz sand respectively. The temperature of the reactor was raised to 90° C., and the reaction pressure was 0.1 MPa. The reactant cyclohexene and acetic acid are respectively fed into the reactor, and the molar ratio of acetic acid to cyclohexene is 4:1. The feed space velocity of cyclohexene is 1.0g.g -1. h -1 . The reaction was run continuously for 250 hours. After the reaction product was collected, the composition of the product was analyzed by gas chromatography. The conversion rate of cyclohexene was 84.2%, and the selectivity of cyclohexyl acetate was 98.2%.
[0073] The addition reaction product is rectified to obtain cyclohexyl acetate with a purity greater than 99.5%. The cyclohexyl acetate was further oxidized, and the specific reaction steps were as fo...
Embodiment 3
[0075] 60ml of macroporous strongly acidic ion-exchange resin Amberlyst36 is packed into the middle part of the stainless steel tubular fixed-bed reactor, and the top and bottom of the catalyst are filled with quartz sand respectively. The temperature of the reactor was raised to 90° C., and the reaction pressure was 0.1 MPa. The reactant cyclohexene and acetic acid are respectively fed into the reactor, and the molar ratio of acetic acid to cyclohexene is 4:1. The feed space velocity of cyclohexene is 1.0g.g -1. h -1 . The reaction was run continuously for 250 hours. After the reaction product was collected, the composition of the product was analyzed by gas chromatography. The conversion rate of cyclohexene was 81.5%, and the selectivity of cyclohexyl acetate was 98.1%.
[0076] The addition reaction product is rectified to obtain cyclohexyl acetate with a purity greater than 99.5%. The cyclohexyl acetate was further oxidized, and the specific reaction steps were as fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com