Drug-loading nanofiber membrane for preventing nasal infection and adhesion and preparation method thereof
A drug-loaded nano-fiber membrane technology, applied in fiber treatment, fiber chemical characteristics, rayon manufacturing, etc., can solve the problems of limited drug-loading capacity and unsatisfactory adhesion effect, so as to avoid adverse reactions, prevent repeated infections, good preventive effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
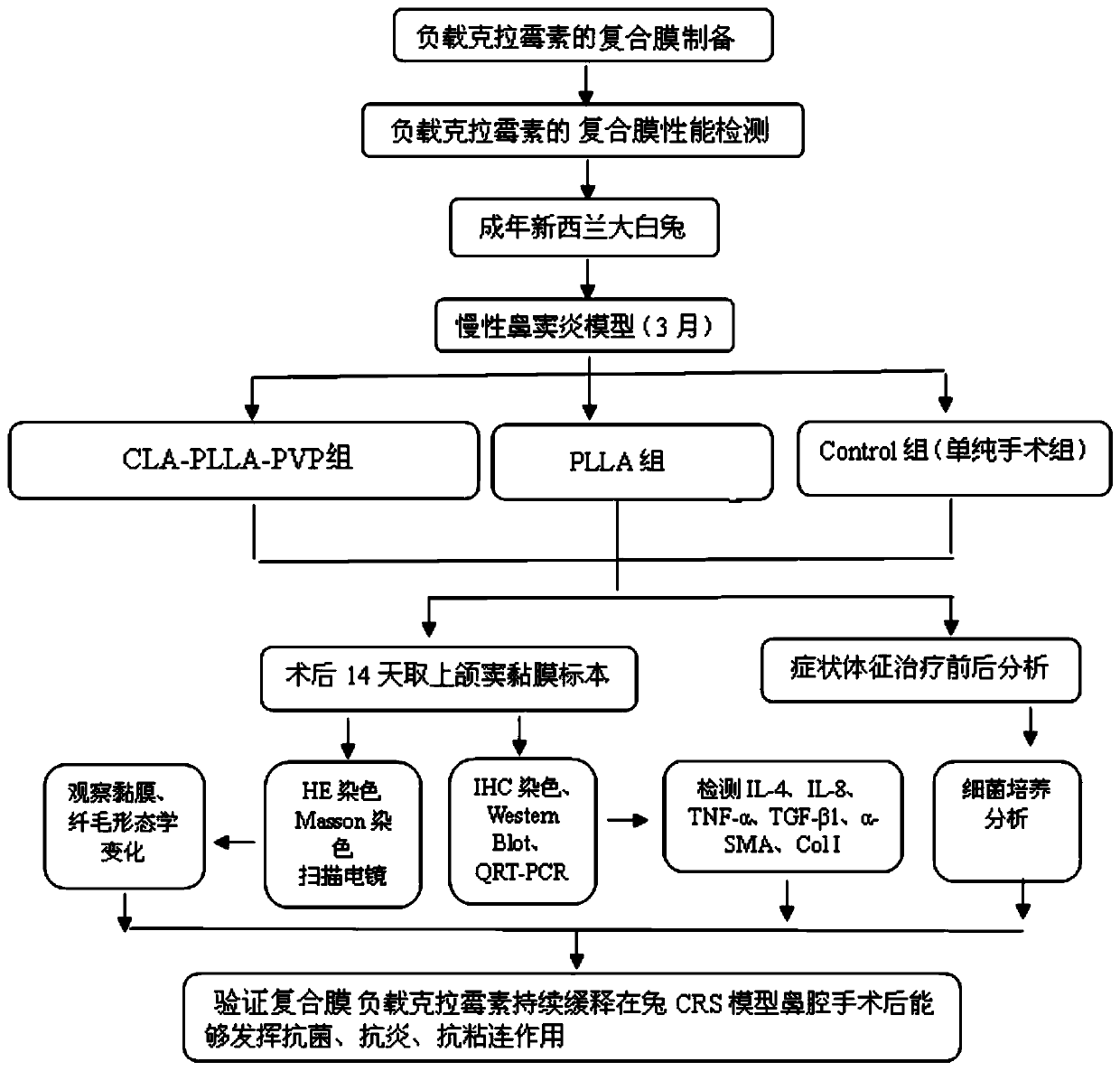
Examples
Embodiment 1
[0055] Preparation of PLLA-PVP membrane loaded with clarithromycin in embodiment 1
[0056] Add 1g PLLA, 0.01g PVP and 0.1g clarithromycin to the mixture of 4g dichloromethane and 2g N,N-dimethylformamide, stir constantly for 1 hour to completely dissolve PLLA and other materials; The spinning solution was sonicated for 10 min in a water bath to eliminate any air bubbles. The mixed solution after sonication was placed in a 10 mL syringe, the diameter of the syringe needle was 0.8 mm, the voltage was fixed at 15 kV, the distance between the needle and the collector was set at 15 cm, and the solution feeding rate was 0.2 mL / Electrospinning was carried out under the condition of 10 minutes, and an electrospun fiber membrane loaded with clarithromycin (CLA-PLLA-PLLA, clarithromycin concentration 10%) was produced.
[0057] And, the PLLA film was produced according to the similar production steps as above; except that only 1 g of PLLA was dissolved in an organic solvent when prep...
Embodiment 2
[0058] Embodiment 2 physical performance detection
[0059] (1) Scanning electron microscope observation
[0060] With prepared PLLA film and CLA-PLLA-PVP film (kind see figure 2 ) was collected on the surface of aluminum foil, followed by vacuum drying for 24 hours, and the morphology of PLLA and CLA-PLLA-PVP fiber membrane was observed by scanning electron microscope.
[0061] image 3Scanning electron micrographs of the PLLA film and the CLA-PLLA-PVP film prepared in Example 1, A is the PLLA film, and B is the CLA-PLLA-PVP film. According to the results of scanning electron microscopy, there is no obvious difference in the fiber morphology of PLLA membrane and CLA-PLLA-PVP membrane. Both of them have smooth surface, similar fiber diameter and porosity. 30% to 95%. It can be seen that the drug loading process of the present invention basically has no effect on the appearance of these fibers.
[0062] (2) Water contact angle measurement
[0063] Water contact angle (wa...
Embodiment 3
[0069] Embodiment 3 in vitro drug experiment
[0070] (1) In vitro drug release ability
[0071] Each 200 mg of CLA-PLLA-PVP fiber membrane was immersed in 50 mL of phosphate-buffered saline (PBS, pH 7.4) solution, placed in a thermostat shaking water bath at a shaking speed of 50 cycles / min. On the 15th, 30th, 45th, and 60th days, 1.0 mL of sustained-release solution medium was taken out for detection, and 1.0 mL of fresh PBS solution was added at the same time. The PBS solution taken out was analyzed by high-performance liquid chromatography (HPLC), and the stationary phase was analyzed at 25°C using an ODS column; the mobile phase was a potassium dihydrogen phosphate solution / methanol (400:600v / v) system; the flow rate was 1.0mL / min, the detection wavelength is 210nm.
[0072] For drug release see Figure 6 , from the drug release curve of the CLA-PLLA-PVP film, it can be seen that clarithromycin can be released slowly and steadily for two months, and a clarithromycin r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com