Drug sustained-release carrier and preparation method and application thereof
A slow-release carrier and drug technology, applied in the direction of pharmaceutical formulations, non-active ingredients medical preparations, medical preparations containing active ingredients, etc., can solve the problems of unreported research and achieve simple preparation process, not easy to dissolve, The effect of not being easily oxidized
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
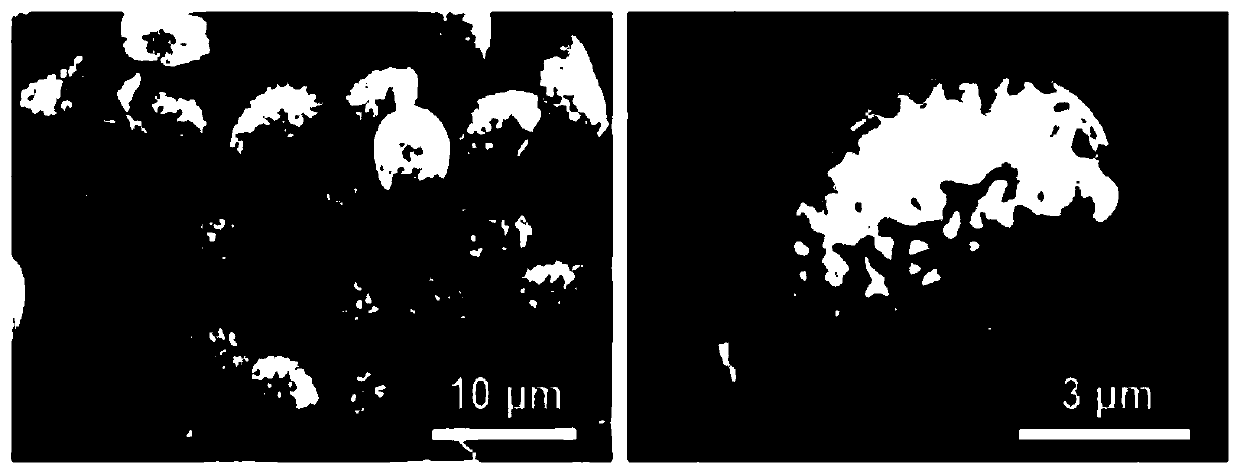
[0043] Concrete steps for preparing assembly (rhodamine B / ganoderma lucidum spore microspheres) by liquid phase transplantation method:
[0044] (1) Take 0.5g ganoderma lucidum spore microsphere sample and place it in a Rhodamine B solution with a concentration of 1mg / mL, and stir at 800rpm / min for 48h at room temperature;
[0045] (2) The stirred mixed solution was centrifuged, the supernatant was discarded, and placed in a constant temperature blast drying oven to dry to obtain the assembled sample assembly Rhodamine B / Ganoderma lucidum spore microspheres.
[0046] Put the rhodamine B / ganoderma lucidum spore microspheres into the dialysis bag, and then put them into the simulated body fluid of the system for release. The specific experimental steps of the in vitro release experiment are as follows:
[0047] (1) Take 0.5g of assembled Rhodamine B / Ganoderma lucidum spore microspheres and put them into a dialysis bag, then put them into an Erlenmeyer flask containing 50mL of si...
Embodiment 2
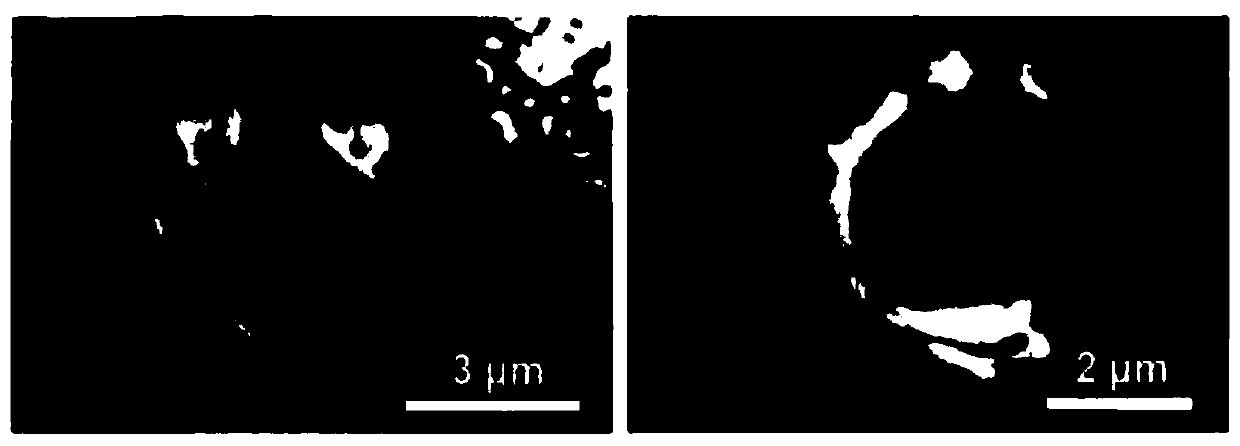
[0052] In this example, the non-steroidal anti-inflammatory analgesic drug ibuprofen was used as a model drug, and the ibuprofen was loaded into the treated ganoderma lucidum spore microspheres by a liquid phase transplantation method. The specific experimental steps are as follows:
[0053] (1) Take 0.5 g of processed Ganoderma lucidum spore microsphere sample, place it in a 250 mL beaker, add 100 mL of ibuprofen / ethanol solution (concentration: 5 mg / mL) into the beaker, and stir at room temperature for 48 h;
[0054] (2) Centrifuge the stirred mixture, filter, wash with absolute ethanol, remove as much as possible the drug molecules loaded on the surface of the ganoderma spore microspheres and dry to obtain the assembly ibuprofen / ganoderma spore microspheres.
[0055] Put the assembled ibuprofen / ganoderma lucidum spore microspheres into the dialysis bag, and then put them into the simulated body fluid of the system for release. The specific experimental steps of the in vitro...
Embodiment 3
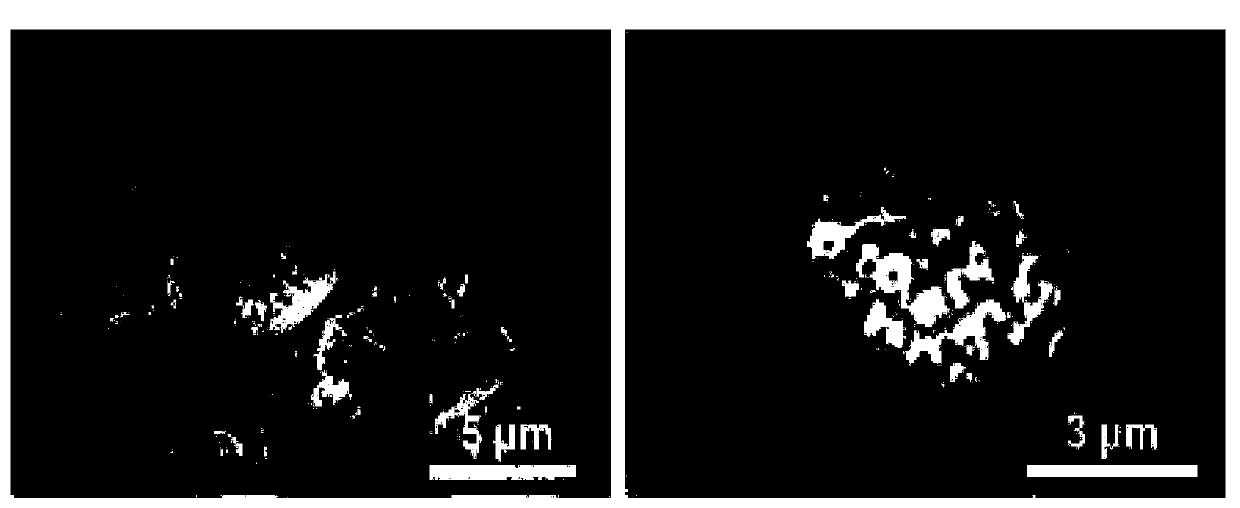
[0061] In this example, the styrene non-steroidal anti-estrogen anti-tumor drug tamoxifen citrate was used as a model drug, and the experiment adopted the liquid phase transplantation method to load the tamoxifen citrate drug into the ganoderma lucidum spore microspheres middle. The specific experimental steps are as follows:
[0062] (1) Grind tamoxifen citrate tablets into fine powder, ultrasonicate in methanol solution until dissolved, centrifuge and filter, take 0.5 g of processed Ganoderma lucidum spore microspheres and place them in 100 mL of tamoxifen citrate / methanol (Concentration is 5mg / mL) solution, stirred at room temperature for 48h;
[0063] (2) Centrifuge the stirred mixture, filter, and dry to obtain the assembled sample assembly tamoxifen citrate / ganoderma spore microspheres.
[0064] Put the assembled tamoxifen citrate / ganoderma lucidum spore microspheres into the dialysis bag, and then put them into the simulated body fluid of the system for release. The s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com