Divalent europium-doped barium fluobromide light-emitting material, preparation method and applications thereof
A luminescent material, barium bromide fluoride technology, applied in luminescent materials, chemical instruments and methods, instruments, etc., can solve the problems of not particularly uniform appearance, large pollution of reduced carbon powder, affecting luminescent performance, etc., to achieve excellent fluorescence and Light-stimulated luminescent properties, high packing density, and uniform size distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] 1) Preparation of precursor material: weigh 89g BaBr 2 and 0.032g EuBr 2 Dissolve in a mixed solution of 2200mL ethanol and 200mL water (ethanol:water=11:1), and stir until completely dissolved to form solution A. Weigh 3.70gNH 4 F was dissolved in a mixed solution of 2200mL ethanol and 200mL water to form solution B. At room temperature and under magnetic stirring, the solution B was slowly added to the solution A for reaction. After the solution B was added dropwise, the reaction was continued for 6 hours. Then centrifuge at 1000rpm / min to obtain a precipitate, then cycle wash with absolute ethanol and methanol, and vacuum dry at 50°C for 5h to obtain the precursor material. The scanning electron microscope image of the precursor material is shown in figure 1 As shown, it can be seen that the particle length is about 80nm, the width is about 60nm, and the thickness is about 30nm.
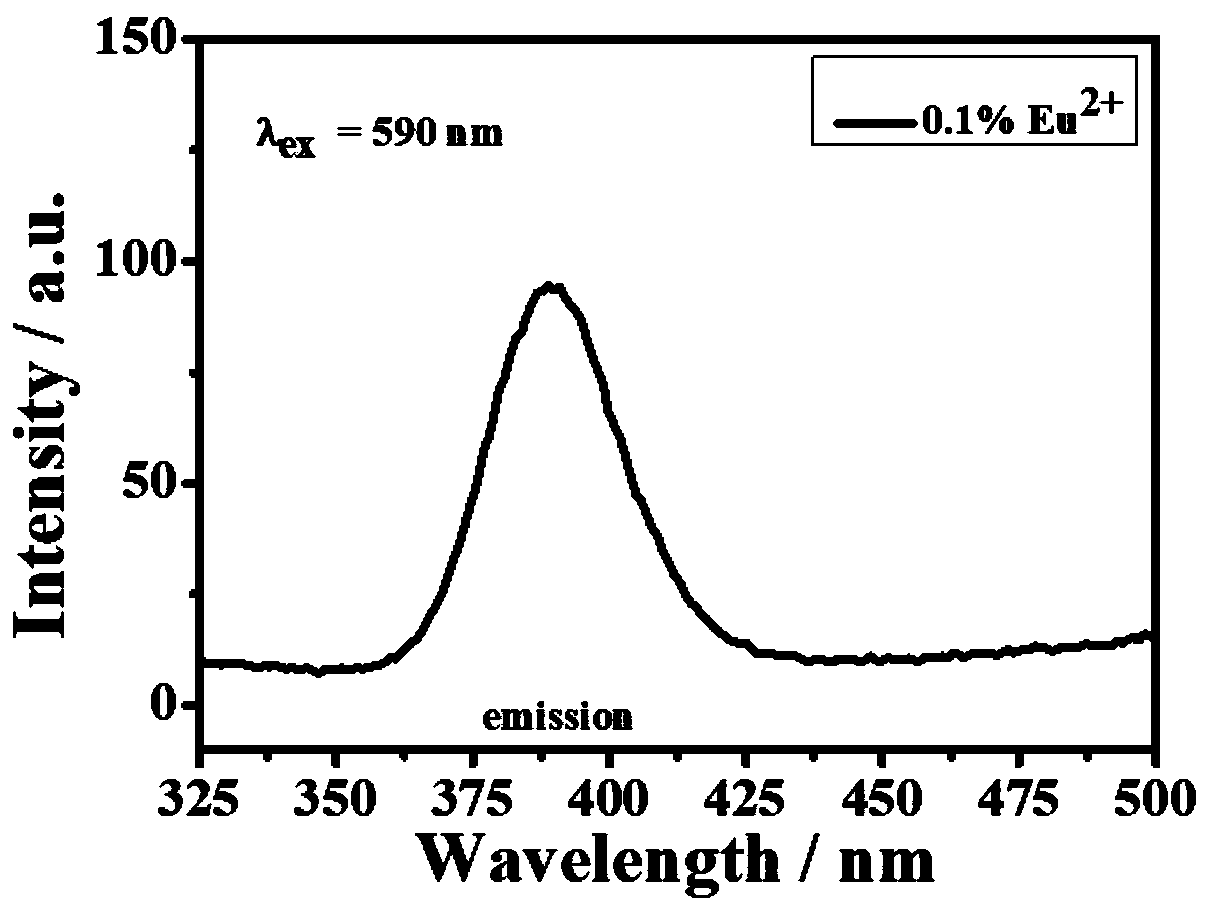
[0046] 2) Luminescent material BaFBr:Eu 2+ Preparation: the precursor material pr...
Embodiment 2
[0049] 1) Preparation of precursor material: weigh 89g BaBr 2 and 0.032g EuBr 2 Dissolve in a mixed solution of 2000mL ethanol and 400mL water (ethanol:water=5:1), and stir until completely dissolved to form solution A. Weigh 3.70gNH 4 F was dissolved in a mixed solution of 2000mL ethanol and 400mL water to form solution B. At room temperature and under magnetic stirring, the solution B was slowly added to the solution A for reaction. After the solution B was added dropwise, the reaction was continued for 6 hours. Then centrifuge at 1000rpm / min to obtain precipitates, then cycle wash with absolute ethanol and methanol, and vacuum dry at 50°C for 5h to form the precursor particles. The particle length is about 500nm, the width is about 400nm, and the thickness is about 50nm.
[0050] 2) Luminescent material BaFBr:Eu 2+ Preparation: the precursor material prepared in step 1) was placed in an alumina crucible, and then placed in a tube furnace for annealing at 600° C. for 2 ...
Embodiment 3
[0053] 1) Preparation of precursor material: weigh 89g BaBr 2 and 0.032g EuBr 2 Dissolve in a mixed solution of 1600mL ethanol and 800mL water (ethanol:water=2:1), and stir until completely dissolved to form solution A. Weigh 3.70gNH 4 F was dissolved in a mixed solution of 1600mL ethanol and 800mL water to form solution B. At room temperature and under magnetic stirring, the solution B was slowly added to the solution A for reaction. After the solution B was added dropwise, the reaction was continued for 6 hours. Then centrifuge at 1000rpm / min to obtain precipitates, then cycle wash with absolute ethanol and methanol, and vacuum dry at 50°C for 5h to form the precursor particles. The particle length is about 1.5 μm, the width is about 1.5 μm, and the thickness is about 100 nm.
[0054] 2) Luminescent material BaFBr:Eu 2+ Preparation: the precursor material prepared in step 1) was placed in an alumina crucible, and then placed in a tube furnace for annealing at 600° C. fo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Width | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com