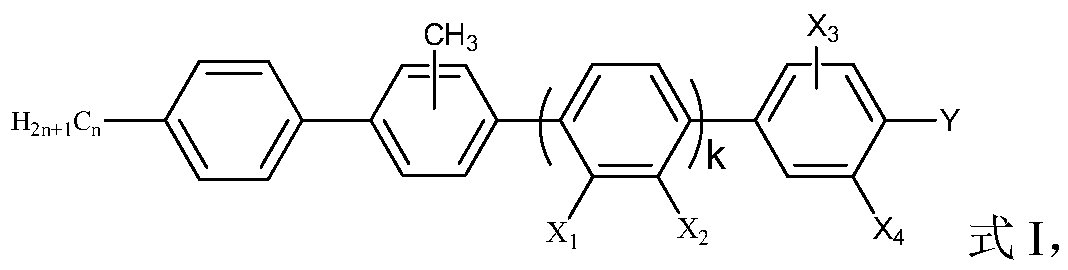
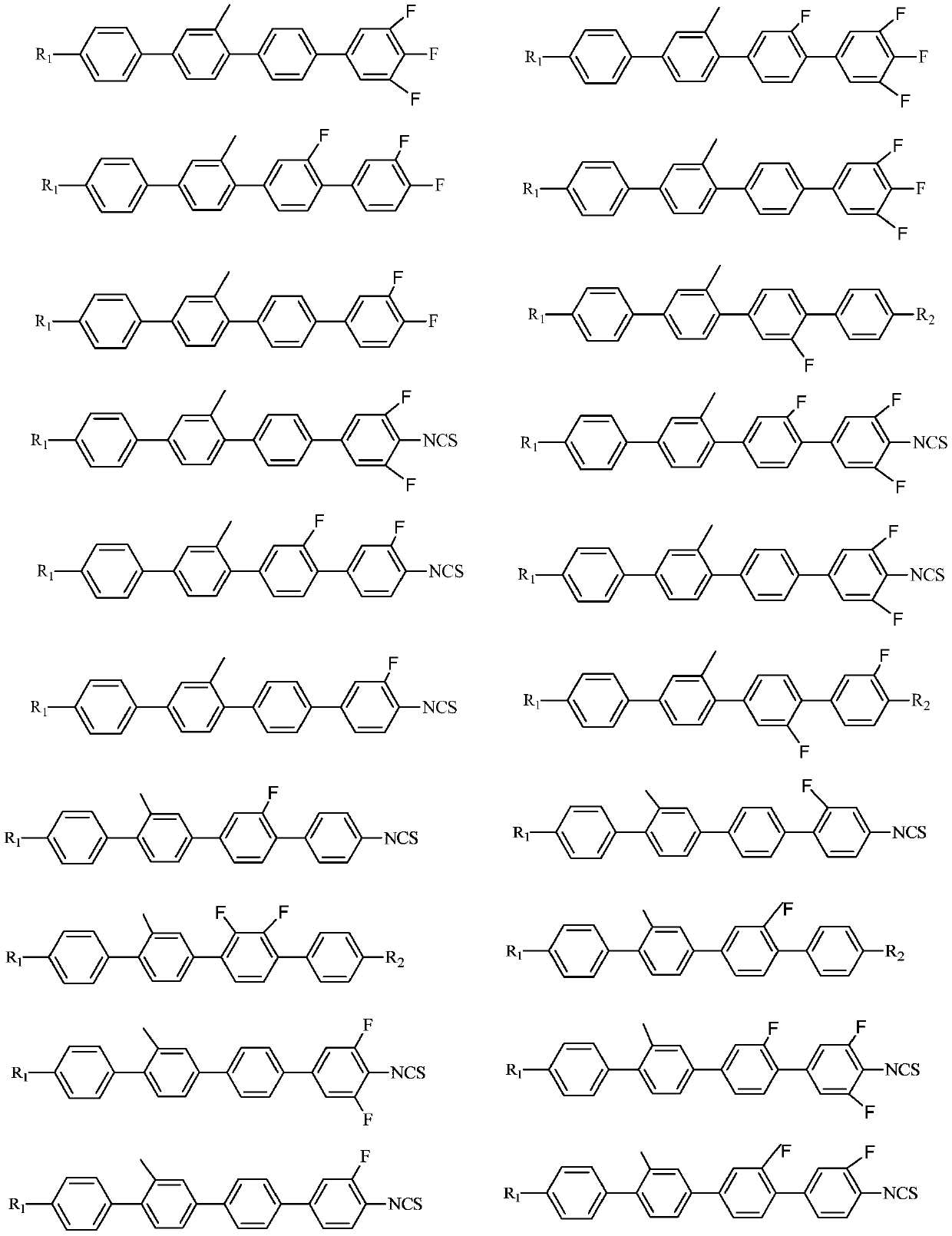
Side methyl polybiphenyl liquid crystal compound, liquid crystal composition and application of composition
A technology of methyl polybiphenyls, liquid crystal compounds, applied in liquid crystal materials, chemical instruments and methods, waveguide-type devices, etc., can solve the problems of large microwave dielectric loss, insufficient Δn value, affecting low temperature performance, etc. The effect of reducing dielectric loss, reducing eutectic point and reducing loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
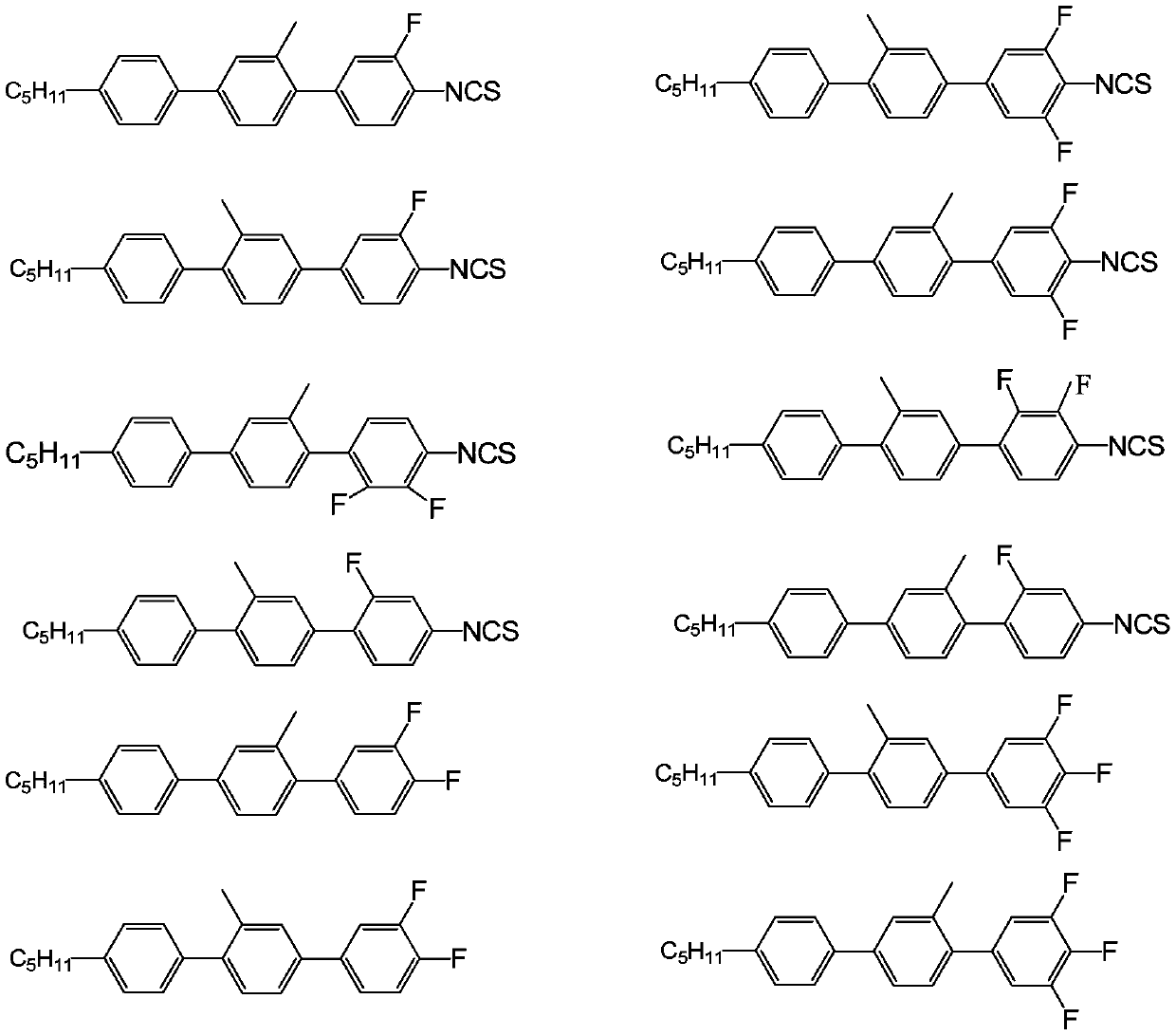
[0062] Synthetic compound (5PP (1) UF), its molecular structure is as follows:
[0063]
[0064] Its synthetic route is as follows:
[0065]
[0066] Its synthesis steps are as follows:
[0067] (1) Add 14.14g (0.132mol) of 2-methylaniline, 33.6g (0.135mol) of iodine, 16.6g (0.166mol) of Anhydrous calcium carbonate and 200ml distilled water, after reacting at room temperature for 1 hour, raise the temperature to 68°C and react for 1 hour; TLC tracking monitoring, stop stirring and heating after the reaction is complete, filter with suction, wash the filter cake with CH2Cl2, and wash the filtrate with CH2Cl2 and Extracted with water until neutral, dried over anhydrous sodium sulfate, filtered with suction, and evaporated the organic solvent to obtain 32g of a purple-black crude product, which was recrystallized with 50% absolute ethanol and 50% water to obtain 27.49g of a white solid a(4- Iodo-2-methylaniline), melting point: 86°C-89°C, yield 89.3%.
[0068] (2) Add 45...
Embodiment 2
[0074] Synthetic compound 5PP (1) GF, its molecular structure is as follows:
[0075]
[0076] The specific synthetic route and method are the same as in Example 1, except that the reactant 3,4,5-trifluorophenylboronic acid in the synthetic step (4) is changed into equimolar 3,4-difluorophenylboronic acid (1.57g, 0.01 mol) can be; the specific dosage is 2-methyl-4'-pentyl biphenyl bromide is added 3.17g (0.01mol), 3,4-difluorophenylboronic acid is added 1.58g (0.01mol), tetrakis (triphenyl Phosphine) palladium was added to 0.12g (1×10 -4 mol), potassium carbonate was added to 5.53g (0.04mol); 1.1g of white solid product 1,2-difluoro-2'-methyl-4"-pentyl terphenyl (5PP(1)GF) was finally obtained; melting point: 33°C-34°C, the reaction yield is 81.4%, and the total synthesis yield is 28.76%. IR(KBr, νmax / cm -1 ): 3445.72, 2927.14, 2858.47, 2359.15, 1608.31, 1488.86, 1414.40, 1309.00, 1266.97, 1221.77, 1182.97, 1115.31, 817.66, 772.97cm -1 ; 1 H-NMR (400MHz, CDCl 3 )δ(ppm)...
Embodiment 3
[0078] Synthetic compound 5PPI (1) UF, its molecular structure is as follows:
[0079]
[0080] The specific synthetic route and method are the same as in Example 1, except that the raw material 2-methylaniline of the synthetic step (1) is changed into equimolar 3-methylaniline (14.14g, 0.132mol); The target product 5PPI (1) UF) of color transparent liquid, reaction yield 84.2%, total synthesis yield is 20.25%. Melting point: -40°C. IR(KBr,νmax / cm -1 ): 3025.74, 2928.34, 2860.95, 1613.68, 1528.95, 1445.87, 1370.18, 1251.51, 1045.07, 824.58, 749.33, 698.99, 572.87cm -1 ; 1 H NMR (400MHz, CDCl 3 )δ(ppm):7.59–7.21(m,9H),2.91–2.77(m,2H),2.52(s,2H),1.86(dd,J=10.1,4.6Hz,2H),1.54(dd,J =25.2,21.6Hz,5H),1.12(t,J=6.7Hz,3H); 13 C NMR (100MHz, CDCl 3 )δ(ppm):142.34,142.08,141.98,139.37,138.04,137.32,136.40,135.47,130.75,128.81,128.49,127.14,125.85,124.26,111.02,110.86,77.16,76.85,35.86,31.83,22.77,20.60, 14.17; 19 F NMR (376MHz, CDCl 3 )δ (ppm): -133.97, -134.03, -162.60, -16...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com