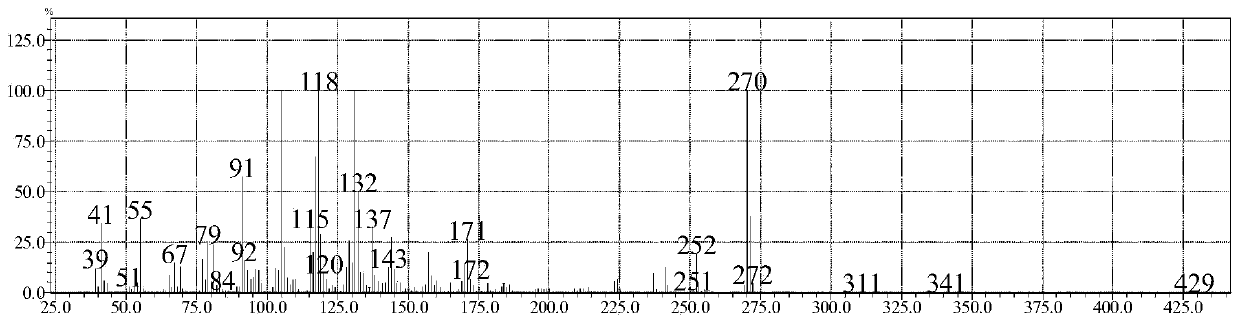
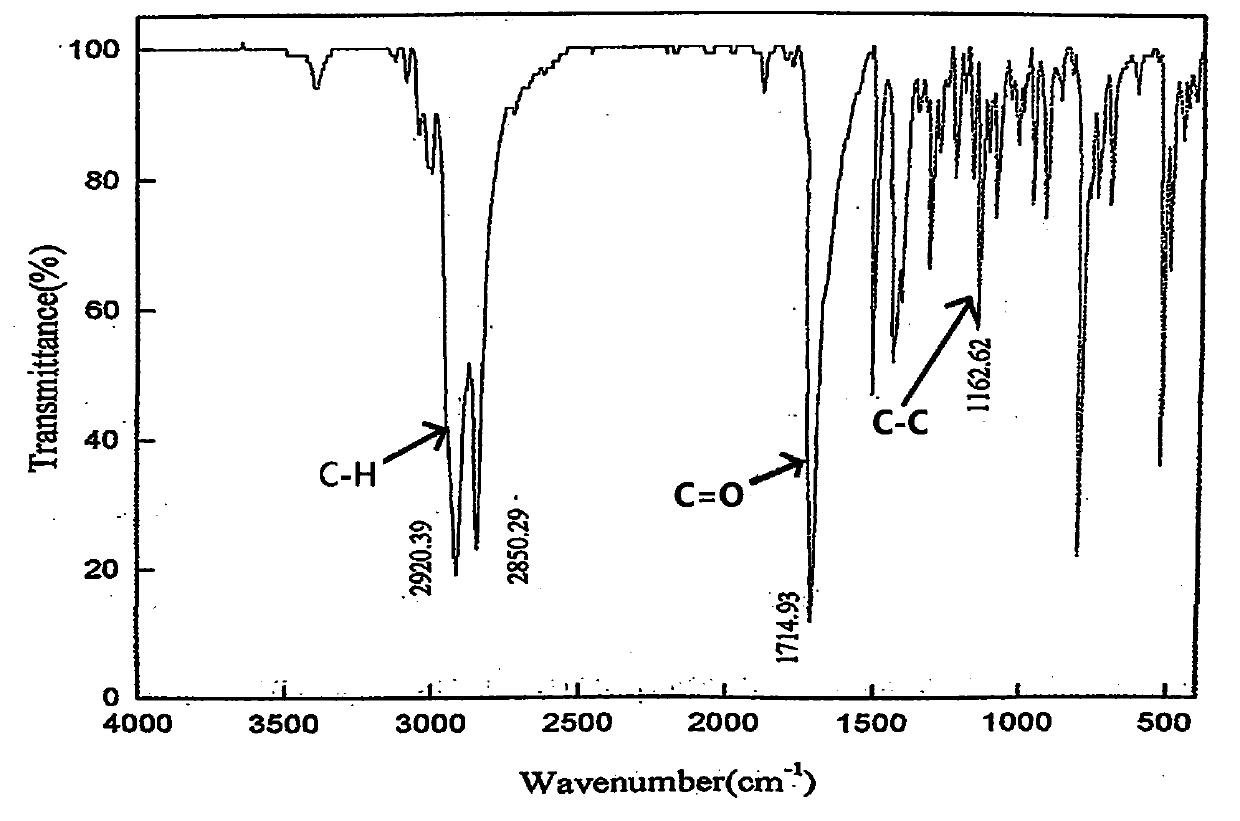
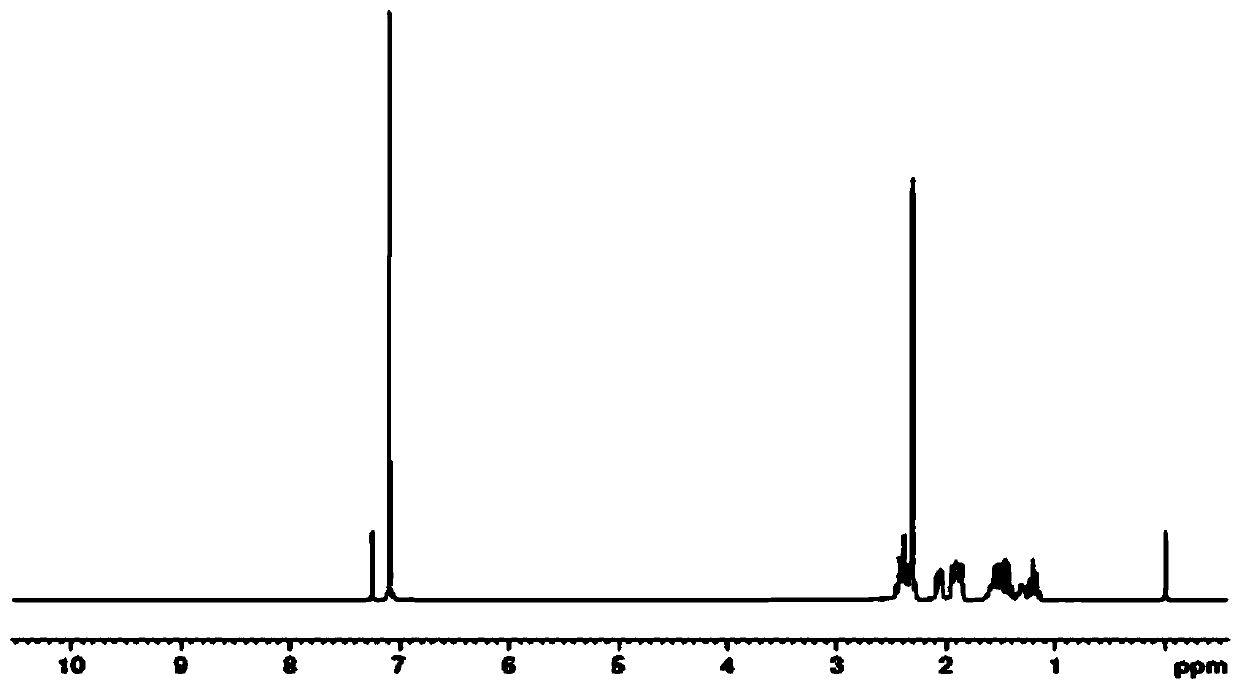
Synthetic method for trans-4'-(4-alkylphenyl)(1,1'-bicyclohexane)-4-one
A technology of bicyclohexane and alkyl phenyl, which is applied in the field of liquid crystal intermediate synthesis, can solve the problems of cumbersome post-processing, many reaction steps, long time consumption, etc., and achieves the effects of saving water washing operation, simplifying operation and low overall cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0041] The invention provides a synthetic method of trans-4'-(4-alkylphenyl)(1,1'-bicyclohexyl)-4-one, comprising the following steps:
[0042] S1: Grignard reaction between 4-alkyl halobenzene and magnesium powder, followed by adding dicyclohexanone ethylene glycol monoketal to generate a mixture containing the compound of formula (II), the reaction equation is as follows:
[0043]
[0044] S2: add acid to the mixture containing the compound of formula (II) in S1 to carry out dehydration and deprotection reaction to generate the compound of formula (III), the reaction equation is as follows:
[0045]
[0046] The mol ratio of described acid and dicyclohexanone ethylene glycol monoketal is 5-15:1, and described acid is hydrochloric acid, sulfuric acid, formic acid or acetic acid;
[0047] S3: The compound of formula (Ⅲ) is hydrogenated under the action of a catalyst to generate the compound of formula (Ⅳ), and the reaction equation is as follows:
[0048]
[0049] S4...
Embodiment 1
[0053] (1) Preparation of a mixture containing a compound of formula (II)
[0054] Under the protection of nitrogen, sequentially add 200ml of tetrahydrofuran and 152.6g of magnesium powder into a dry 10L three-necked flask, and start stirring. The system is heated to 57°C, 0.07g of iodine is added, and 19.4g of 4-methylchlorobenzene is added dropwise to trigger Grignard After the initiation is successful, add 390g of methyl chlorobenzene diluted with 823ml of tetrahydrofuran dropwise. During the dropping process, the temperature is controlled at 70°C. Hexanone ethylene glycol monoketal and 5060ml of toluene solution, the dropwise addition is completed, after 1 hour of heat preservation at 60°C, the reaction is stopped, and the reaction solution is directly used for the next step reaction, GC>95.0%, the yield of this step is calculated as 100% ;
[0055] (2) Preparation of intermediate formula (Ⅲ) compound
[0056] Under the protection of nitrogen, add 1484g of 70% sulfuric ...
Embodiment 2
[0062] (1) Preparation of a mixture containing a compound of formula (II)
[0063] Under the protection of nitrogen, sequentially add 240ml of tetrahydrofuran and 68.7g of magnesium powder into a dry 10L three-necked flask, and start stirring. The system is heated to 55°C, 0.07g of iodine is added, and 41.5g of 4-methylchlorobenzene is added dropwise to trigger a Grignard reaction. , after successful initiation, add dropwise 200g of methyl chlorobenzene diluted with 2174ml of tetrahydrofuran, and control the temperature at 60°C for weak reflux during the dropping process. The tetrahydrofuran solution, after the dropwise addition is completed, if the reaction is refluxed at 70°C for 1 hour, the reaction is stopped, and the reaction solution is directly used for the next step reaction, GC>98.0%, and the yield of this step is calculated as 100%;
[0064] (2) Preparation of intermediate formula (Ⅲ) compound
[0065] Under the protection of nitrogen, add 1934.5g of 30% hydrochlori...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com