Preparation method of hemostatic menstruation-regulating granules
A granulation and menstruation regulating technology, which is applied in the direction of medical preparations with non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, etc., can solve the problems of difficult operation of granulation process, unfavorable storage, easy bed collapse, etc., to achieve The process is simple, the production cycle is shortened, and the integrity is good
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] One embodiment of the present invention provides a preparation method of hemostasis regulating menstruation granule, comprising the following steps S1-S6.
[0037] S1. Take astragalus, donkey-hide gelatin, codonopsis pilosula, white peony root, angelica, Agrimony, madder, bergamot, Dipsacus and auxiliary materials according to the formula of Zhixue Tiaojing granule.
[0038] In one embodiment, the Hemostasis Tiaojing Granules include the following raw materials in parts by weight: 45-50 parts of astragalus, 45-50 parts of donkey-hide gelatin, 43-48 parts of Codonopsis pilosula, 12-16 parts of white peony root, 10-50 parts of angelica 15 parts, 45-50 parts of agrimony, 20-28 parts of madder, 12-16 parts of bergamot, 12-16 parts of Dipsacus, 10-20 parts of pregelatinized starch and 25-30 parts of sucrose powder.
[0039] Further, in terms of parts by weight, the raw materials of Zhixue Tiaojing Granules include 48 parts of astragalus, 48 parts of donkey-hide gelatin, 45...
Embodiment 1
[0065] 1) Raw materials: Astragalus 48kg, Ejiao 48kg, Codonopsis 45kg, Paeoniae Alba 15kg, Angelica 12kg, Agrimony 48, Rubia 24kg, Bergamot 12kg, Dipsacus 12kg, Precrossed starch 18kg and sucrose powder 27kg.
[0066] 2) Extraction of volatile oil from angelica and bergamot medicinal materials: Add 6 times the amount of water to angelica and bergamot, steam distillation and extraction for 4 hours, collect volatile oil, keep the mixed water extract of angelica and bergamot for later use, and keep the dregs of angelica and bergamot for later use ; Take β-cyclodextrin of 8 times the amount of the volatile oil, add an appropriate amount of purified water to mix, and adjust it into a paste, dilute the volatile oil with 4 times the amount of 95% ethanol, use a colloid mill to clathrate for 20 minutes, filter, and store at 50°C Dry at ~60°C to obtain β-cyclodextrin inclusion compound.
[0067] 3) Take half the amount of Astragalus membranaceus and dry it on a belt dryer at 70°C to 80...
Embodiment 2
[0074] Step 1) to step 4) of Embodiment 2 are the same as Embodiment 1.
[0075] 5) Pump the extract into a concentrator to concentrate to an extract with a specific gravity of 1.35 (measured at 60°C).
[0076] 6) Put the extract into a baking tray for vacuum drying, spread it evenly, control the temperature at 60°C-80°C, vacuum degree at -0.06--0.08MPa, and dry for 15 hours to obtain dry extract, powder it, and obtain the particle size It is 150 μm~250 μm, and the dry extract powder of water content is 6.0wt%, about 58kg.
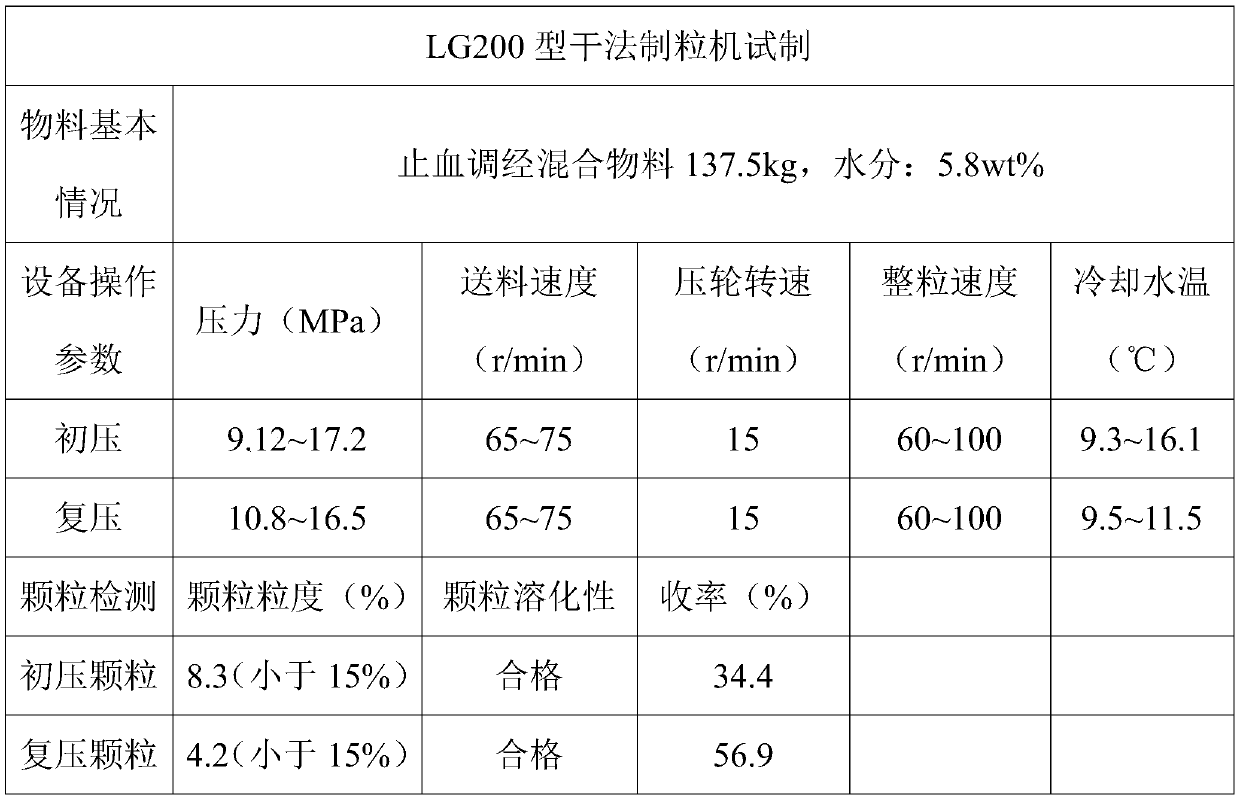
[0077] 7) Take the crushed dry extract powder, donkey-hide gelatin powder, and sucrose powder, add astragalus ultrafine powder, and pregelatinized starch, mix evenly, and then pump it to the hopper of LG200 dry granulator through a vacuum feeder, and pass through the horizontal Feeding, the speed of horizontal feeding is 75r / min; to the pressing roller, the pressure is 13MPa, the operating speed of the pressing roller is 15r / min, and the displacement of the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com