A kind of preparation method of granisetron intermediate
A technology of intermediates and schemes, applied in the field of preparation of granisetron intermediates, can solve the problems of high cost, low product purity and yield, and high equipment and operational requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] 1. Synthesis of Pseugranicine (Ⅳ)
[0042] Add 0.44kg Na in 3L water 2 HPO 4 And 0.09kg of methylamine hydrochloride, add 0.2kg of acetone dicarboxylic acid, cool to about 0°C, add 0.25kg of glutaraldehyde dropwise, keep at 0°C and stir for 1h, warm up to 25°C and stir for 24h. Adjust the pH to about 3 with 0.2L concentrated hydrochloric acid (concentration 36%), raise the temperature to 75° C. and stir for 1 hour, cool to room temperature, and adjust the pH to above 10 with a 50% NaOH solution. After extraction with DCM, the solvent was spun off to obtain a crude product. Add 0.4kg of neutral alumina with 0.2L methyl tert-butyl ether under stirring, heat to reflux, drop the crude product, stir and reflux for 0.5h, naturally cool to room temperature, filter, and use petroleum ether: methyl tert-butyl ether = The filter cake was rinsed three times with 3:1 solvent. The filtrate was spin-dried to obtain 0.16 kg pseudopusicine (IV).
[0043] 2. Synthesis of 3-Homotropine oxi...
Embodiment 2
[0049] Steps 1-2 are the same as in Example 1, the difference is:
[0050] 3. Synthesis of 3α-High Tropine Amine (Ⅰ)
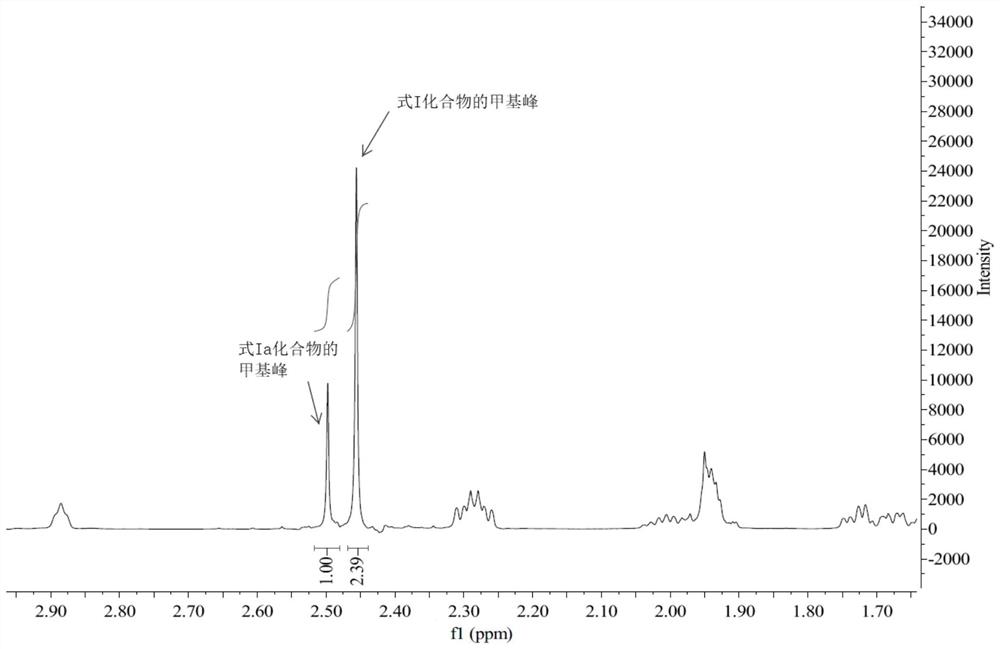
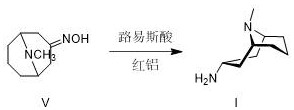
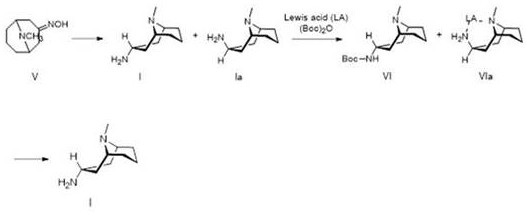
[0051] Add 445ml of 70% Red-Al to 320ml of THF, add 306.8g of titanium tetrachloride dropwise, control the temperature within 0±5℃, stir for 2h, add dropwise 136g of 3-high tropinone oxime, control the temperature at 0±5℃, after dripping, warm to room temperature and react for 8h. Add 20% NaOH aqueous solution, separate the layers, wash the organic phase with saturated ammonium chloride and saturated sodium chloride, dry the organic phase with anhydrous sodium sulfate, concentrate the organic phase under reduced pressure, and collect the residue by distillation under reduced pressure 92~96 At a temperature of 10 mmHg, 81.5 g of 3α-homotropane (I) was obtained. Purity 89.9%, yield: 65.4%.
[0052] 1 H NMR(400MHz, CDCl 3 )δ3.2(m,1H),3.0(d,J=6.0Hz,2H), 2.45(s,3H), 2.29(m,2H), 1.94(m,3H), 1.79(br,6H), 1.46 (m, 1H), 1.13 (m, 2H), 0.98 (m, 2H).
Embodiment 3
[0054] Steps 1-2 are the same as in Example 1, the difference is:
[0055] 3. Synthesis of 3α-High Tropine Amine (Ⅰ)
[0056] Add 500ml of 70% Red-Al to 320ml of THF, add 89.2g of concentrated sulfuric acid (98%) dropwise, control the temperature within 0±5℃, stir for 2h, add dropwise 152g of 3-hotropine oxime, control The temperature is at 0±5°C, after the addition is completed, the temperature is raised to room temperature and reacted for 8 hours. Add 20% NaOH aqueous solution, separate the layers, wash the organic phase with saturated ammonium chloride and saturated sodium chloride, dry the organic phase with anhydrous sodium sulfate, concentrate the organic phase under reduced pressure, and collect the residue by distillation under reduced pressure 92~96 At a temperature of 10mmHg, 104.9 g of 3α-homotropane (I) was obtained. Purity 90.5%, yield: 75.3%.
[0057] 1 H NMR(400MHz, CDCl 3 )δ3.2(m,1H),3.0(d,J=6.0Hz,2H), 2.45(s,3H), 2.29(m,2H), 1.94(m,3H), 1.79(br,6H), 1.46 (m, 1H),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com