A ruthenium-based ammonia synthesis catalyst with nitrogen-doped porous carbon material as carrier and preparation method thereof
A nitrogen-doped porous carbon, ruthenium-based catalyst technology, applied in chemical instruments and methods, ammonia preparation/separation, physical/chemical process catalysts, etc., can solve the problems to be further improved, high specific surface area, etc. Effects of specific surface area and strength, high specific surface area, and high ammonia synthesis activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
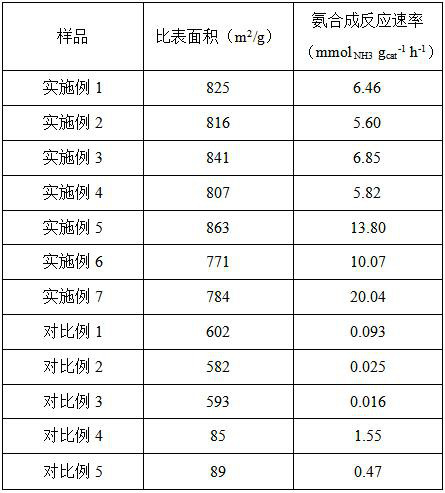
Examples
Embodiment 1
[0028] 1) Dissolve 1.93 g zinc nitrate hexahydrate in 48 ml sodium hydroxide solution (6 mol / L);
[0029] 2) Dissolve 2.104 g of 2-methylimidazole in 9.6 ml of N,N-dimethylformamide (purity 99.5%), and then add 6.4 ml of sodium hydroxide (6 mol / L) solution;
[0030] 3) After mixing and stirring the solutions obtained in the above steps (1) and (2), they were heated and reacted in a hydrothermal kettle at a constant temperature of 120 °C for 4 hours to obtain a milky white solution, which was washed with methanol solution and centrifuged to obtain a solid product , and then dried at a constant temperature in a vacuum oven at 80°C for 12 hours to obtain a solid product;
[0031] 4) Put the solid product obtained in the above step (3) into a tube furnace, and in 10% (volume fraction) H 2 - Under the Ar atmosphere, the temperature was increased at a rate of 2 ℃ / min to 800 ℃ for 4 hours for high-temperature carbonization, and nitrogen-doped porous carbon materials were obtained; ...
Embodiment 2
[0035] 1) Dissolve 1.26 g zinc nitrate hexahydrate in 30 ml sodium hydroxide solution (6 mol / L);
[0036] 2) Dissolve 2.104 g of 2-methylimidazole in 15 ml of N,N-dimethylformamide (purity 99.5%), and then add 6 ml of sodium hydroxide (6 mol / L) solution;
[0037] 3) Mix and stir the solution obtained in the above steps (1) and (2) evenly, then heat and react at a constant temperature of 130 ℃ for 4 hours in a hydrothermal kettle to obtain a milky white solution, wash with methanol solution, and centrifuge to obtain a solid product , and then dried at a constant temperature in a vacuum oven at 70°C for 12 hours to obtain a solid product;
[0038] 4) Put the solid product obtained in the above step (3) into a tube furnace, and in 10% (volume fraction) H 2 - Under the Ar atmosphere, the temperature was raised to 700 °C at a heating rate of 2 °C / min for 5 hours for high-temperature carbonization, and nitrogen-doped porous carbon materials were obtained;
[0039]5) Dissolve 12.52...
Embodiment 3
[0042] 1) Dissolve 1.93 g zinc nitrate hexahydrate in 48 ml sodium hydroxide solution (6 mol / L);
[0043] 2) Dissolve 2.104 g of 2-methylimidazole in 9.6 ml of N,N-dimethylformamide (purity 99.5%), and then add 6.4 ml of sodium hydroxide (6 mol / L) solution;
[0044] 3) After mixing and stirring the solutions obtained in the above steps (1) and (2) evenly, heat and react in a hydrothermal kettle at a constant temperature of 140 °C for 4 hours to obtain a milky white solution, wash with methanol solution, and centrifuge to obtain a solid product , and then dried at a constant temperature in a vacuum oven at 80°C for 10 hours to obtain a solid product;
[0045] 4) Put the solid product obtained in the above step (3) into a tube furnace, in pure H 2 Nitrogen-doped porous carbon materials were obtained by heating at a rate of 2 °C / min to 900 °C for 3 hours in the atmosphere for high-temperature carbonization;
[0046] 5) Dissolve 12.52 ml of ruthenium nitrosyl nitrate solution (r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com