Method for preparing diosgenin by hydrolysis of ternary two-phase AlCl3
A technology of diosgenin and aluminum trichloride, which is applied in the directions of organic chemistry, steroids, etc., can solve the problems of low saponin yield, complicated process and mechanism, and many by-products, etc., so as to improve the saponin yield, The effect of shortening the extraction cycle and reducing the amount of acid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
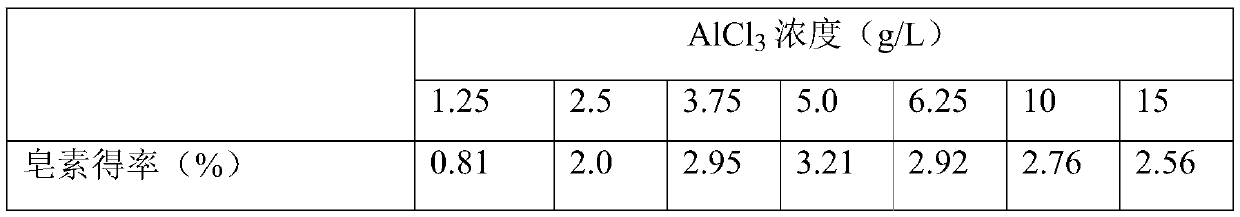
[0027] Accurately weigh 5.0g Dioscorea chrysanthemum powder and put it into a 300mL Parr reactor, then add 80mL 4.0g / LAlCl 3 A ternary biphasic solution composed of aqueous solution, 75mL of ethanol and 100mL of petroleum ether was stirred evenly, and the reaction kettle was set at a rotation speed of 150rpm, and reacted at an extraction temperature of 150°C for 2.0h. After the end, the diosgenin extract in the petroleum ether organic phase was rotary evaporated, concentrated, crystallized, and dissolved in chromatographic grade methanol for sample preparation. Dioscin was then analyzed and quantified. The bottom wastewater solution is collected for subsequent COD analysis.
[0028] The analysis method of diosgenin is as follows: Dilute the above-mentioned diosgenin extract dissolved in constant volume in methanol to a certain number of times, filter it with a 0.22 μm filter membrane, collect it into a 1 mL sample bottle, and prepare a sample for later use. The saponin stand...
Embodiment 2
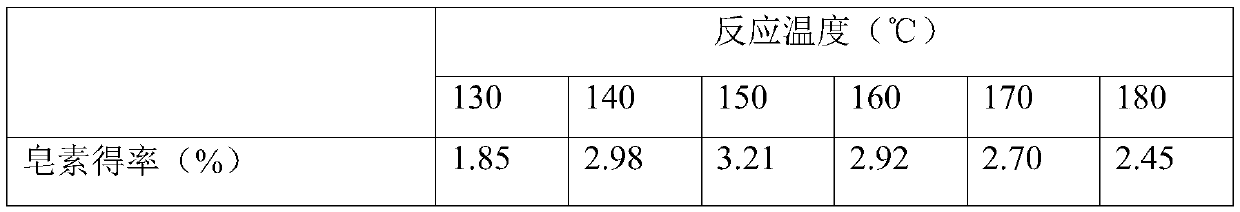
[0031] This example examines the influence of temperature on the yield of saponin in the ternary two-phase system. Accurately weigh 5.0g Dioscorea chrysanthemum powder and put it into a 300mL Parr reactor, then add 80mL 5.0g / L AlCl 3 The ternary biphasic solution composed of aqueous solution, 75mL ethanol and 100mL petroleum ether is stirred evenly, and the rotation speed of the reaction kettle is set to 150rpm. , 170°C, 180°C) and start timing, the reaction time is 2.0h. After the end, the diosgenin extract in the petroleum ether organic phase was rotary evaporated, concentrated, crystallized, and dissolved in chromatographic grade methanol for sample preparation. According to the method analyzed in Example 1, the yield of diosgenin from chrysanthemum leaf is calculated, and the effect of temperature on AlCl in the ternary two-phase system is investigated. 3 Influence of extraction yield of saponin. The results are shown in Table 1:
[0032] Table 1
[0033]
[0034] ...
Embodiment 3
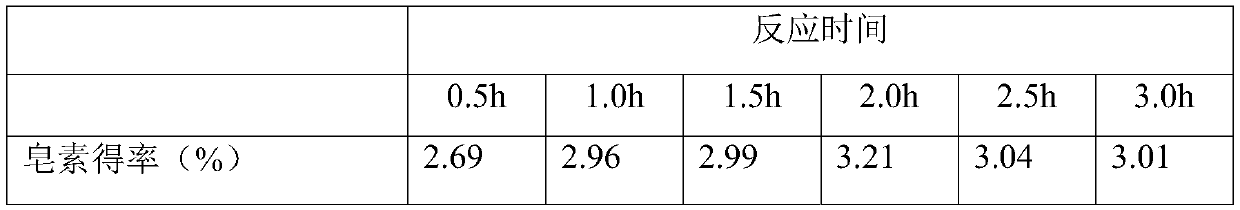
[0036] This example examines the influence of extraction time on the yield of saponin in the ternary two-phase system. Accurately weigh 5.0g Dioscorea chrysanthemum powder and put it into a 300mL Parr reactor, then add 80mL 5.0g / L AlCl 3 The ternary biphasic solution composed of aqueous solution, 75mL ethanol and 100mL petroleum ether was stirred evenly, and the reaction kettle was set at a rotation speed of 150rpm, and reacted respectively at an extraction temperature of 150°C (0.5h, 1.0h, 1.5h, 2.0h, 2.5h, 3.0h). After the end, the diosgenin extract in the petroleum ether organic phase was rotary evaporated, concentrated, crystallized, and dissolved in chromatographic grade methanol for sample preparation. According to the method analyzed in Example 1, the yield of diosgenin from chrysanthemum leaf is calculated, and the effect of reaction time on AlCl in the ternary two-phase system is investigated. 3 Influence of extraction yield of saponin. The results are shown in Tab...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com