The method for extracting iridium from alkaline cyanide solution by 2-ethylhexyltributylphosphine bromide
A technology of hexyltributylphosphine bromide and ethyl bromide, which is applied in the field of precious metal hydrometallurgy, can solve problems that have not been reported in the literature, and achieve the effects of short extraction cycle, stable performance and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
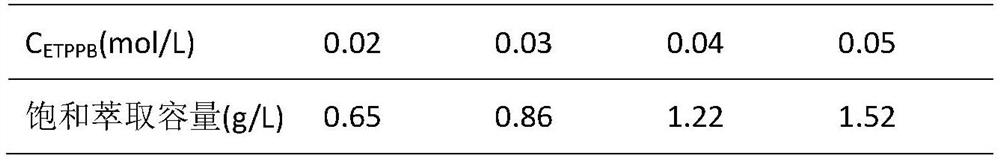
[0033] Get the Ir(CN) whose Ir(III) concentration is 51.2mg / L 6 3- 90 mL of the solution was placed in a separatory funnel, and the pH value of the solution was adjusted to 9.0 as the aqueous phase of the extraction system. The organic phase was prepared, wherein the total volume of the organic phase was 30 mL, the organic phase contained 3 mL of diisooctyl sulfoxide (DIOSO), the balance was chloroform as a diluent, and the concentration of ETPPB in the organic phase was 0.02 mol / L. Add the organic phase to the water phase (O / A=1:3), mechanically oscillate for 2 minutes and let it stand for stratification. After phase separation, measure the concentration of iridium in the water phase, and obtain the iridium concentration in the organic phase by subtraction. Take the organic phase loaded with iridium, put it into another separatory funnel, add NaBr with a concentration of 0.2mol / L according to the ratio between the organic phase and the aqueous phase (O / A=3:1) for back extrac...
Embodiment 2
[0035] Get the Ir(CN) whose Ir(III) concentration is 75.3mg / L 6 3- 80 mL of the solution was placed in a separatory funnel, and the pH value of the solution was adjusted to 9.5 as the aqueous phase of the extraction system. The organic phase was prepared, wherein the total volume of the organic phase was 40 mL, the organic phase contained 6 mL of trioctylphosphine oxide (TOPO), the remainder was diluent with cyclohexane, and the concentration of ETPPB in the organic phase was 0.02 mol / L. The organic phase was added to the water phase (O / A=1:2), mechanically oscillated for 4 minutes and allowed to stand for stratification. After phase separation, the concentration of iridium in the water phase was measured, and the concentration of iridium in the organic phase was obtained by subtraction. Take the organic phase loaded with iridium, put it into another separatory funnel, add NaBr at a concentration of 0.3mol / L according to the ratio of the organic phase to the aqueous phase (O / ...
Embodiment 3
[0037] Get the Ir(CN) whose Ir(III) concentration is 101.2mg / L 6 3-50 mL of the solution was placed in a separatory funnel, and the pH of the solution was adjusted to 10.0 as the aqueous phase of the extraction system. The organic phase was prepared, wherein the total volume of the organic phase was 50 mL, the organic phase contained 10 mL of trialkylphosphine oxide (TRPO), the balance was sulfonated kerosene as a diluent, and the concentration of ETPPB in the organic phase was 0.03 mol / L. The organic phase was added to the water phase (O / A=1:1), mechanically oscillated for 5 minutes and allowed to stand for stratification. After phase separation, the concentration of iridium in the water phase was measured, and the concentration of iridium in the organic phase was obtained by subtraction. Take the organic phase loaded with iridium, put it into another separatory funnel, add 0.4mol / L NaBr according to the ratio between the organic phase and the aqueous phase (O / A=1:1) for bac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com