Novel idarubicin hydrochloride compound
A technology of idarubicin hydrochloride and its compound, which is applied in the field of medicine to achieve the effects of controllability, reduction of manufacturing costs and costs, and ease of preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
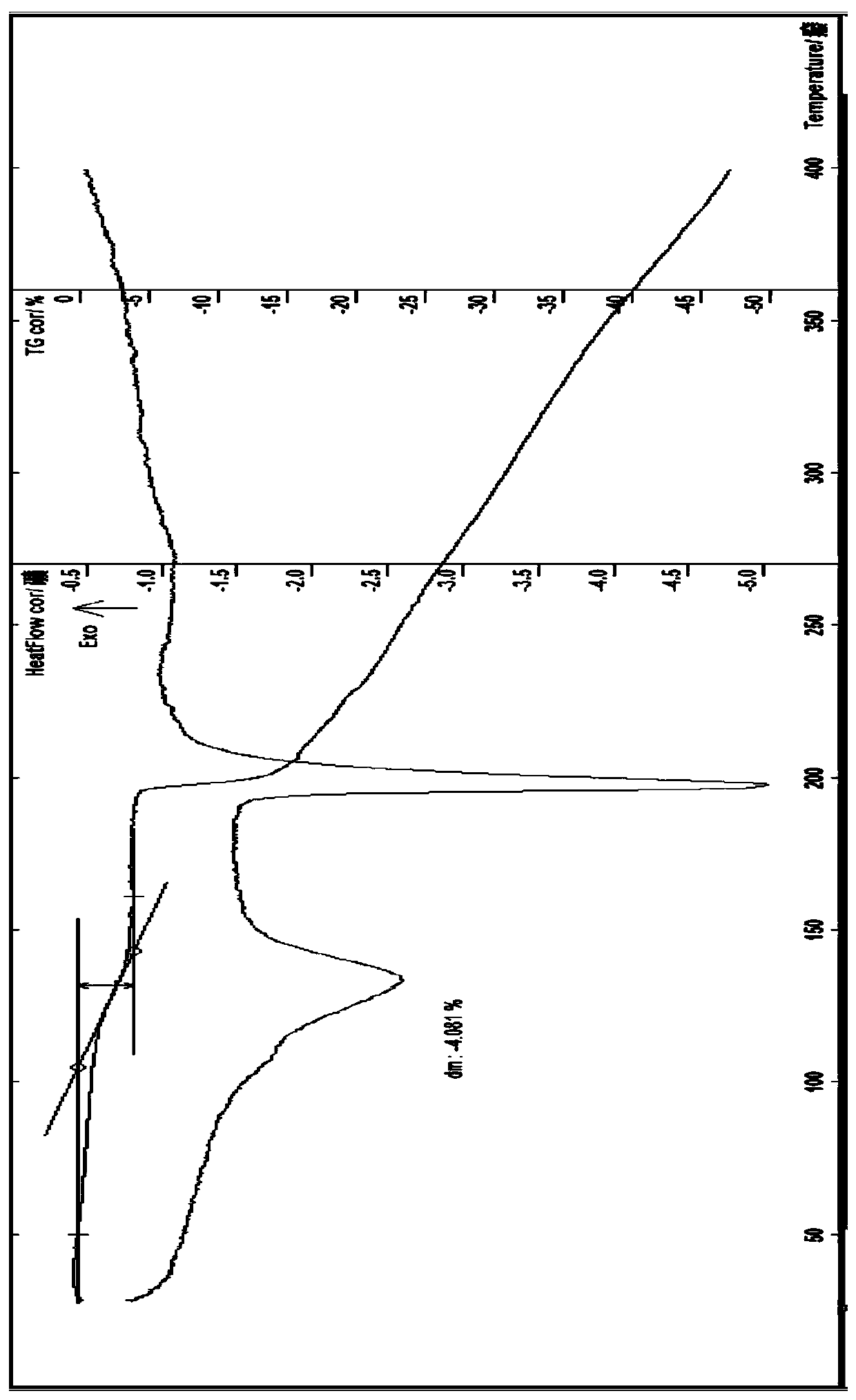
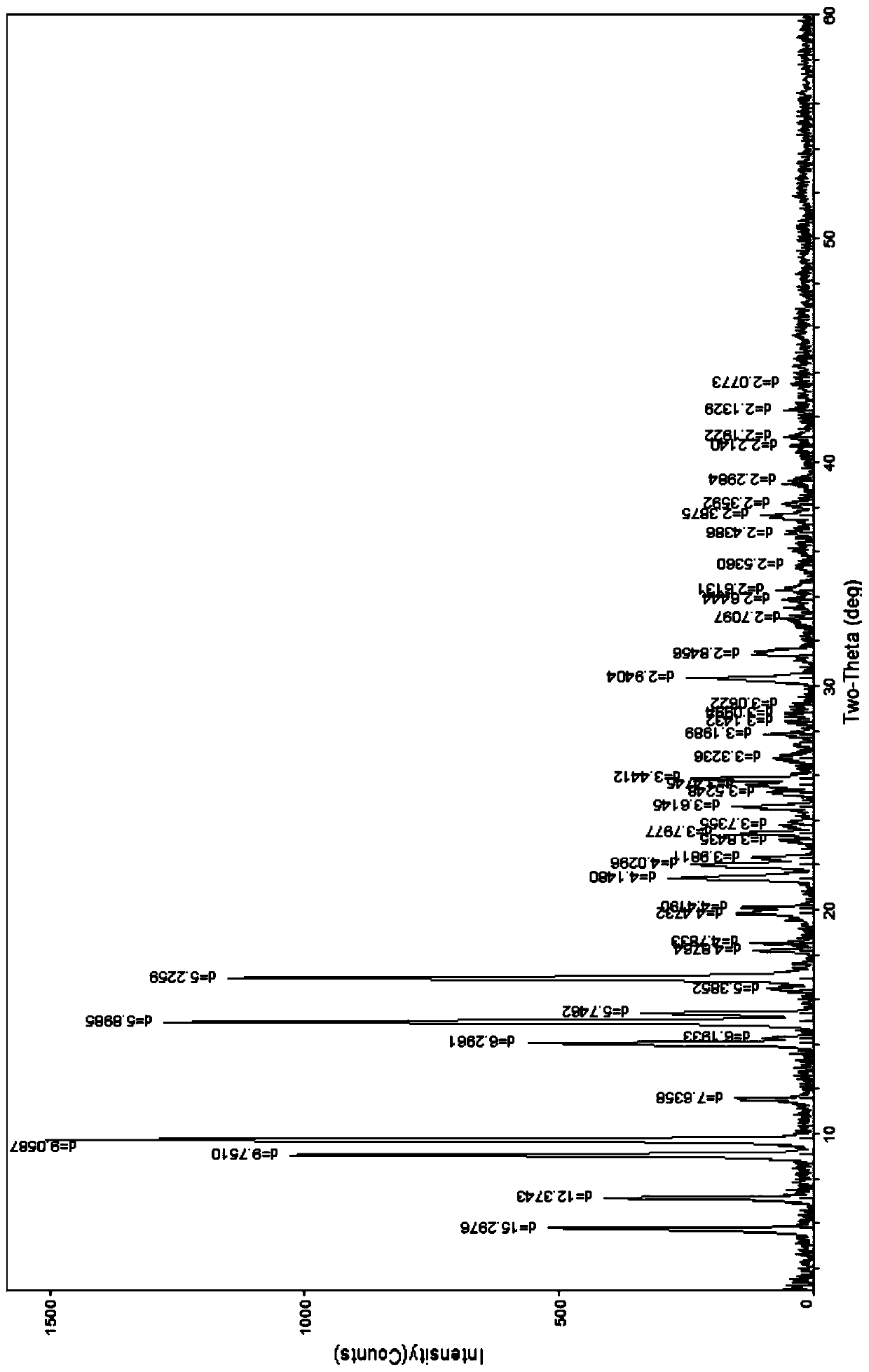
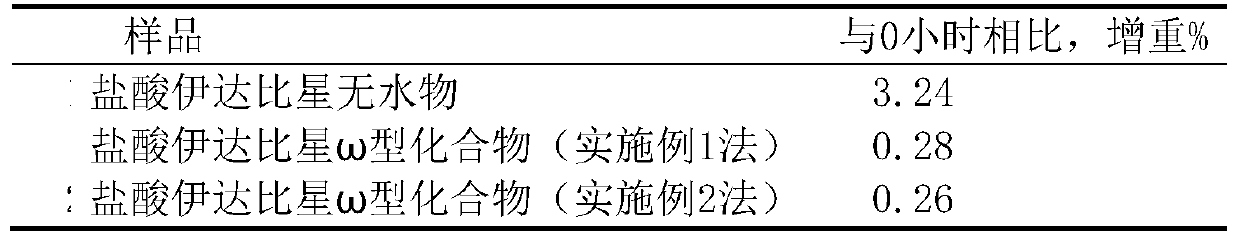
[0128] The preparation of embodiment 1 idarubicin hydrochloride 1.25 hydrate (ω type compound)
[0129] Add 5 g of idarubicin and 10 ml of methanol into a 250 ml three-necked flask, stir, and protect with nitrogen, add an appropriate amount of dichloromethane, heat up to 30-40 ° C, stir to dissolve, add 4M ethanol hydrochloric acid solution at room temperature to adjust the solution The pH value is about 3.0, stir for 20 minutes, evaporate part of the solvent under reduced pressure, then add 20ml of absolute ethanol, place at 0°C, wait until the precipitate is fully separated, filter with suction, wash with a small amount of ethanol, and filter with suction, the obtained solid is diluted in an oven for 40 Blow dry at about ℃ for 3 hours, and obtain about 4.2 g of orange-red crystals; Identification: ①Take about 10 mg of this crystal, add 0.5 ml of nitric acid to dissolve, add 0.5 ml of water, burn with flame for 2 minutes, let cool, add dropwise silver nitrate for test Liquid,...
Embodiment 2
[0130] The preparation of embodiment 2 idarubicin hydrochloride 1.25 hydrate (ω type compound)
[0131]Add 8g of idarubicin hydrochloride anhydrous to a 250ml three-necked flask, add an appropriate amount of methanol, protect with nitrogen, stir, heat up to about 50-63°C, stir until completely dissolved, add 60ml of acetonitrile, cool to below 5°C and place , when the precipitate is fully separated, filter with suction, wash with a small amount of ethanol twice, and filter with suction, put the obtained solid in a flask, add appropriate amount of methanol and water (95:5), heat and stir to dissolve, add acetonitrile and ethanol (1:1) 150ml Carry out recrystallization, place it below 5°C until the crystals are fully separated, filter with suction, wash the solid with a small amount of ethanol, filter with suction, dilute the obtained solid in an oven at 35°C for about 3 hours, and dry at about 50°C for 1 hour to obtain Orange-red crystalline solid 5.2g; Identification: ①Take 10...
Embodiment 3
[0132] Embodiment 3 Preparation of idarubicin hydrochloride crystalline hydrate freeze-dried powder injection (prescription: 1000 bottles)
[0133] Prescription: idarubicin hydrochloride crystalline hydrate (prepared by the method of embodiment 1 or embodiment 2, the main ingredient weight is calculated in idarubicin hydrochloride) 10g, sorbitol 40g, disodium EDTA 0.05g, lactic acid of about 1M and Disodium hydrogen phosphate solution, appropriate amount, appropriate amount of water for injection;
[0134] Take the prescribed amount of idarubicin hydrochloride crystalline hydrate, sorbitol, and disodium EDTA in the liquid preparation tank, add about 1000ml of water for injection, stir to dissolve, and adjust the pH to 4.5 with about 1M lactic acid and disodium hydrogen phosphate solution ~5.5, add activated carbon 0.01% (W / V) and stir for 30 minutes, filter with 0.22 micron microporous membrane, pack according to the weight of main drug 10mg / bottle, freeze at -45~-30℃ for abou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com