A kind of dimethoxycurcumin polymer micelle and its preparation method and medical application
A technology of dimethoxycurcumin and polymer glue, which is applied in the field of dimethoxycurcumin polymer micelles and its preparation, can solve the problem of low drug loading and encapsulation efficiency, far-flung requirements, and poor quality. Poor controllability and other problems, to achieve the effect of improving bioavailability, uniform distribution, and reducing leakage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] The total synthesis preparation of embodiment 1 dimethoxycurcumin
[0042] Dimethoxyturmeric crude drug was prepared with reference to the total synthesis process route provided by the US patent (US005679864A). The preparation process is briefly described as follows. Methyl vanillin (23.3 g), boron oxide (4.66 g), anhydrous dimethylformamide (90 mL), pentanedione (7.2 mL) and dimethoxypropane (17.2 mL) were put into 250 mL three-neck flask, tightly stoppered, shake evenly and store in N 2Slowly raise the temperature to 70°C under protection, and when the solids are completely dissolved, start to slowly add n-butylamine (2.8 mL) dropwise, finish adding within about 120 minutes, continue to react for about 1 hour, and pour the reaction solution into a 400 mL boiling 5% acetic acid solution, stir evenly, after naturally cooling to room temperature, add 200 mL deionized water, shake well, let stand in 4°C refrigerator for 2 days, pour off the upper layer solution, add ace...
Embodiment 2
[0044] The semi-synthetic preparation of embodiment 2 dimethoxycurcumin
[0045] Take curcumin (10 g) and put it in a 250 mL three-necked bottle, add 1:1 ether-methanol mixed solution (v / v, 80 mL), shake well, add sodium hydroxide (3.2 g), heat to reflux under stirring, Iodomethane (15 g) was added dropwise within 30 min, and the reaction was monitored by TLC until the starting material was consumed. Remove the solvent under reduced pressure, add ammonia water (60 mL) to precipitate a large number of yellow crystals, filter, wash with water until neutral, dry and perform silica gel column chromatography, and use petroleum ether-ethyl acetate (8: 2, v / v ) mixed solvent system for elution, collected and combined the yellow fractions in the middle section, removed the solvent under reduced pressure, and dried in vacuo to obtain an orange-yellow powdery solid (3.5 g). The results of spectrum analysis were consistent with the product prepared by total synthesis, and it was iden...
Embodiment 3
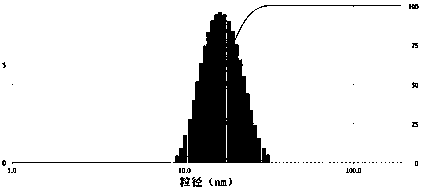
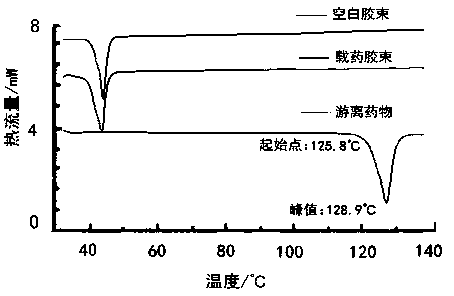
[0046] Example 3 Preparation of dimethoxycurcumin polymer micelles and freeze-dried powder thereof
[0047] (1) BOC-phenylalanine end-capped polyethylene glycol monomethyl ether-polycaprolactone block copolymer (mPEG 2000 -PCL 1800 -BP) / DMC micelles and its lyophilized powder
[0048] Weigh 180 mg of the copolymer polymer and 20 mg of dimethoxycurcumin, put them in a 50 mL eggplant-shaped bottle, add 15 mL of absolute ethanol, and ultrasonically dissolve in a water bath at 50°C until the solution is clear and free of insoluble matter. Ethanol was removed by pressure distillation to form a uniform film on the bottom and inner wall of the bottle. Inject 10 mL of warm purified water, hydrate in a water bath at 50°C for 45 min, until the drug film is completely peeled off and dissolved, add 80 mg of mannitol, filter the resulting solution through a 0.22 μm filter membrane, and freeze-dry to obtain dimethoxyturmeric Freeze-dried powder of drug-loaded micelles. The theoretical d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com