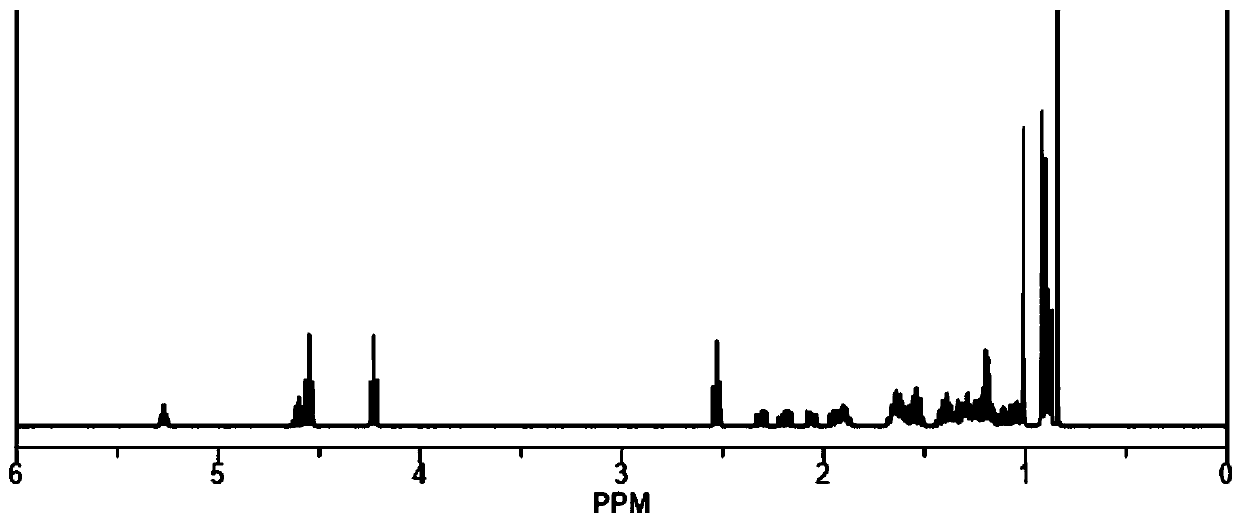
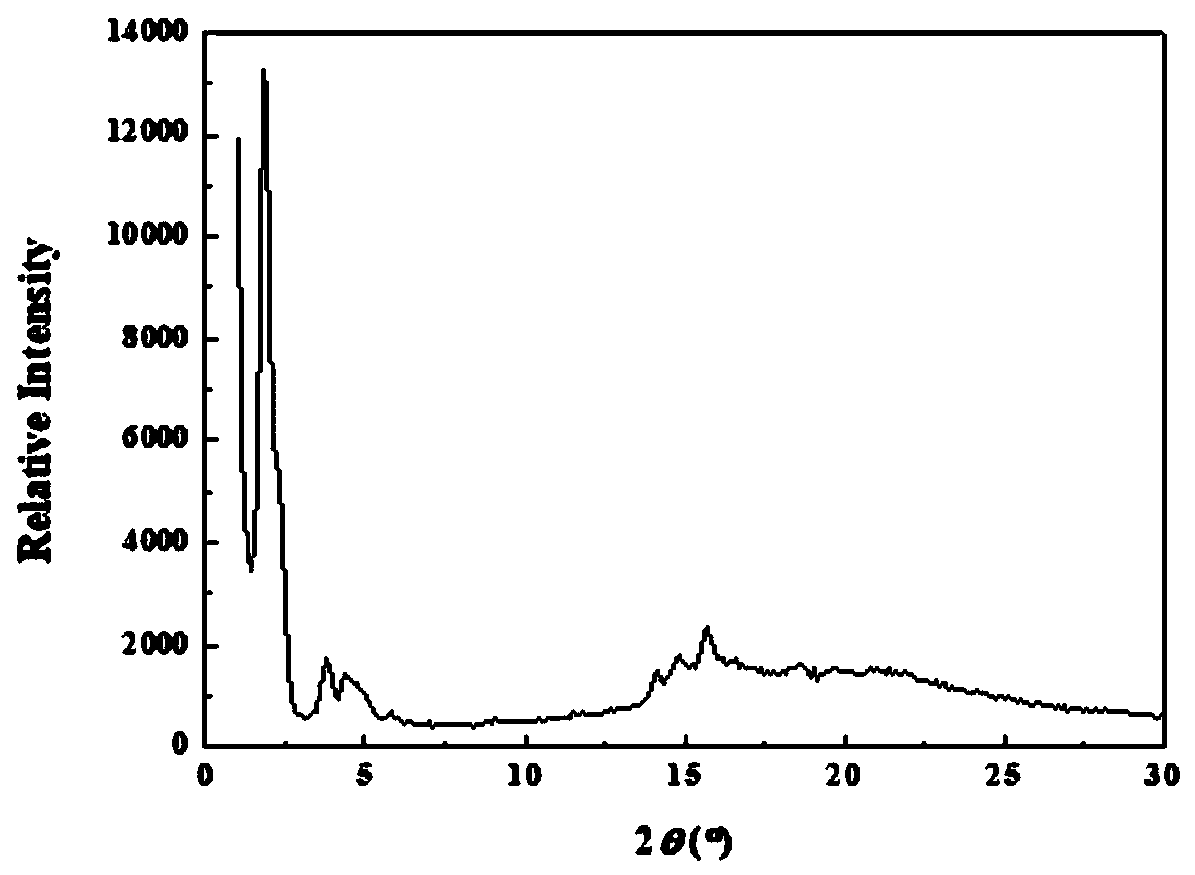
Main chain type biodegradable liquid crystal polymer and preparation method thereof
A liquid crystal polymer, biodegradable technology, applied in the direction of liquid crystal materials, chemical instruments and methods, etc., to achieve the effect of improving biocompatibility, broadening the scope of application, and good liquid crystal performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0069] 1. Preparation of end group protected alkanoic acid
[0070] 1.1. Dissolve hydroxy-terminated alkanoic acid or terminal amino acid in a reactor filled with dichloromethane and triethylamine solution, wherein the molar ratio of hydroxy-terminated alkanoic acid or terminal amino acid to triethylamine is 1: (1~10) ;
[0071] 1.2. Under the cooling condition of ice-water bath, slowly add the dichloromethane solution of triphenylchloromethane dropwise under stirring, the amount of triphenylchloromethane added is the molar ratio of terminal hydroxy alkanoic acid or terminal amino acid 1: (1~10 ) times;
[0072] 1.3. After dropping, react at room temperature for 6-12 hours;
[0073] 1.4, sequentially water, 1mol L -1 Wash with hydrochloric acid, saturated sodium bicarbonate solution, and saturated brine, dry over anhydrous sodium sulfate, concentrate to dryness, and recrystallize with petroleum ether to obtain terminal-protected alkanoic acid.
[0074] 2. Preparation of te...
Embodiment 1
[0112] In this embodiment, the main chain type biodegradable liquid crystal polymer and its preparation method are as follows:
[0113] (1) Add 18g (200mmol) of 3-hydroxypropionic acid in dichloromethane (300mL) and 83mL (600mmol) of triethylamine into the reaction flask, cool in an ice-water bath, and slowly add 55.8g of triphenylchloromethane dropwise under stirring (200 mmol) in dichloromethane (200 mL). After dropping, react at room temperature for 8h. Sequentially water, 1mol L -1 Wash with hydrochloric acid, saturated sodium bicarbonate solution, and saturated brine, dry over anhydrous sodium sulfate, concentrate to dryness, and recrystallize with petroleum ether to obtain white end-group-protected propionic acid. Yield: 73%, melting point: 163-165°C.
[0114] (2) Add a solution of 16.6g (50mmol) of end-protected propionic acid in dichloromethane (300mL) and 80mL of dimethylformamide (DMF), cool in an ice-water bath, and slowly add 16.9mL of oxalyl chloride dropwise u...
Embodiment 2
[0130] In this embodiment, the main chain type biodegradable liquid crystal polymer and its preparation method are as follows:
[0131] (1) Add 13.2g (100mmol) of 6-hydroxycaproic acid in dichloromethane (300mL) and 55mL (400mmol) of triethylamine into the reaction flask, cool in an ice-water bath, slowly add 55.8% triphenylchloromethane dropwise under stirring g (200 mmol) in dichloromethane (200 mL). After dropping, react at room temperature for 8h. Sequentially water, 1mol L -1 Wash with hydrochloric acid, saturated sodium bicarbonate solution, and saturated brine, dry over anhydrous sodium sulfate, concentrate to dryness, and recrystallize with petroleum ether to obtain white end-group-protected caproic acid. Yield: 62%, melting point: 117-119°C.
[0132] (2) Add a solution of 18.7g (50mmol) of end-protected hexanoic acid in dichloromethane (300mL) and 80mL of methylformamide (DMF), cool in an ice-water bath, slowly add 16.9mL of oxalyl chloride dropwise under stirring ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com