Recombinant pectinase, gene thereof, recombinant vector, preparation method and application
A pectinase and gene technology, applied in the field of biomolecule cloning, can solve problems such as being unsuitable for mass and low-cost production of pectinase, unsuitable for mass production of recombinant pectinase, inactivation of target products, etc. Safety, reducing the metabolic load of host cells, and achieving mass production effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] This embodiment provides an optimized artificially synthesized recombinant pectinase gene with a 6×His tag at the C-terminus. The specific sequence is shown in SEQ ID No.1 in the sequence listing, and the protein sequence corresponding to the gene As shown in SEQID No.2 in the sequence listing. The optimized DNA sequences were compared by NCBI, and there was no obvious similarity.
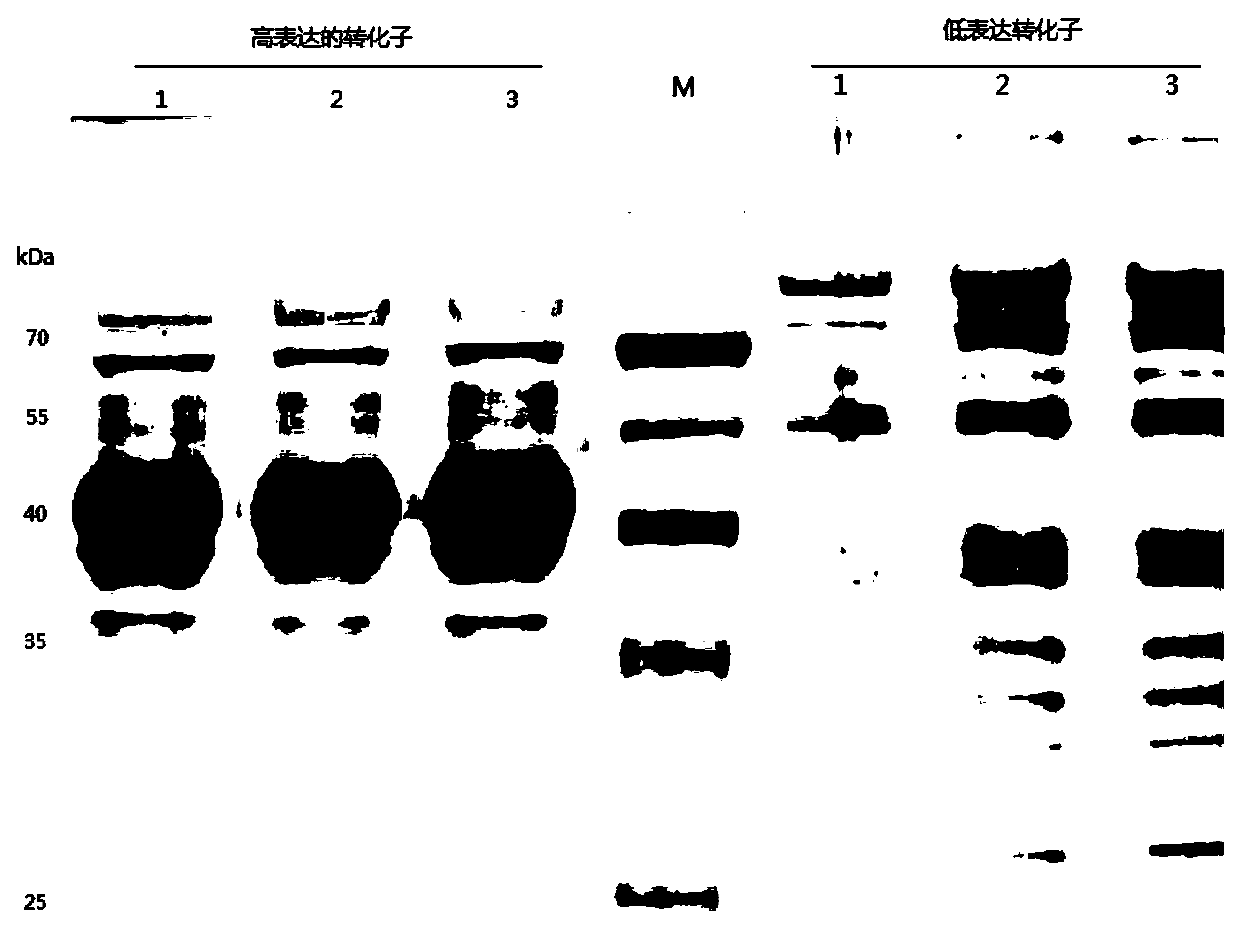
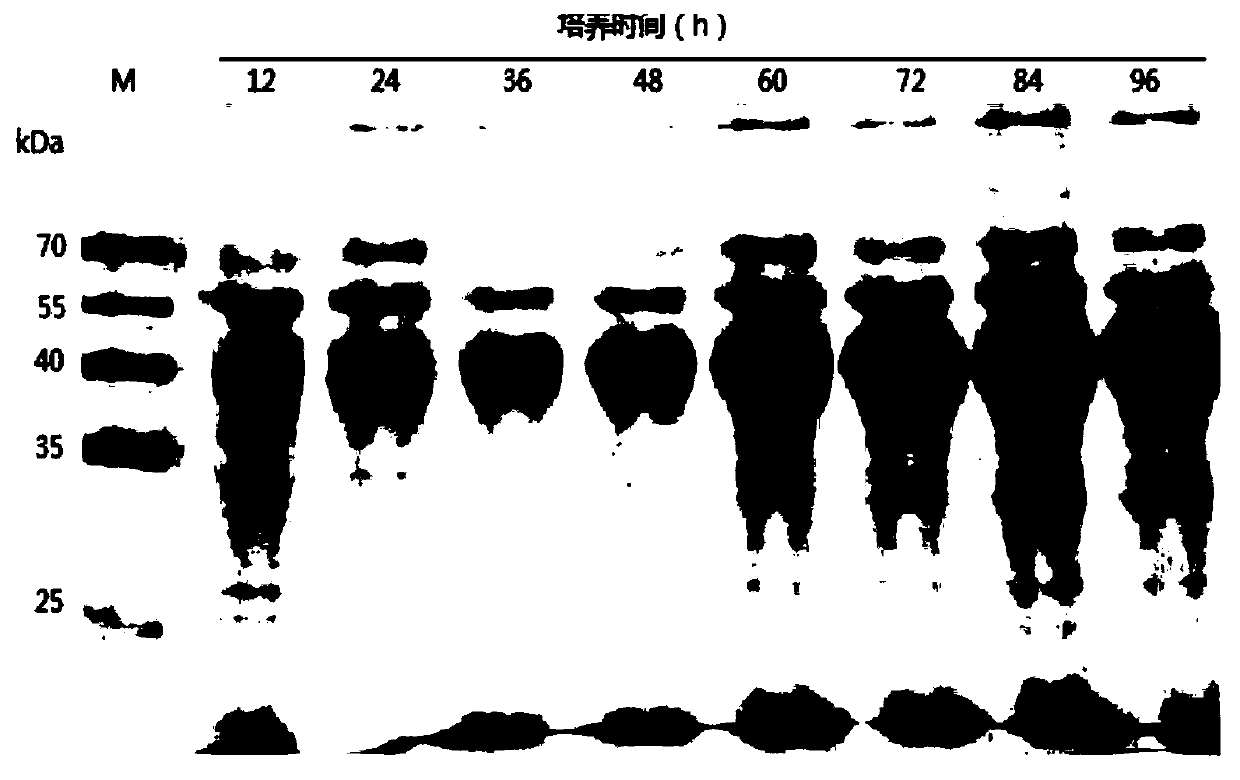
[0046] The present invention synthesizes the DNA sequence shown in SEQ ID No.1 according to the sequence characteristics of the pectinase gene itself and the yeast codon preference, the natural DNA of the pectinase before optimization, and the synthesized pectinase after optimization according to the codon preference of Escherichia coli The artificial DNA sequences were connected to the Pichia pastoris secretory expression vector pGAPZαA to obtain the recombinant vectors, and then the recombinant vectors were transformed into the Pichia pastoris host strain X-33 by using the lithium chloride...
Embodiment 2
[0048] This embodiment provides a method for preparing protein, which specifically includes the following steps:
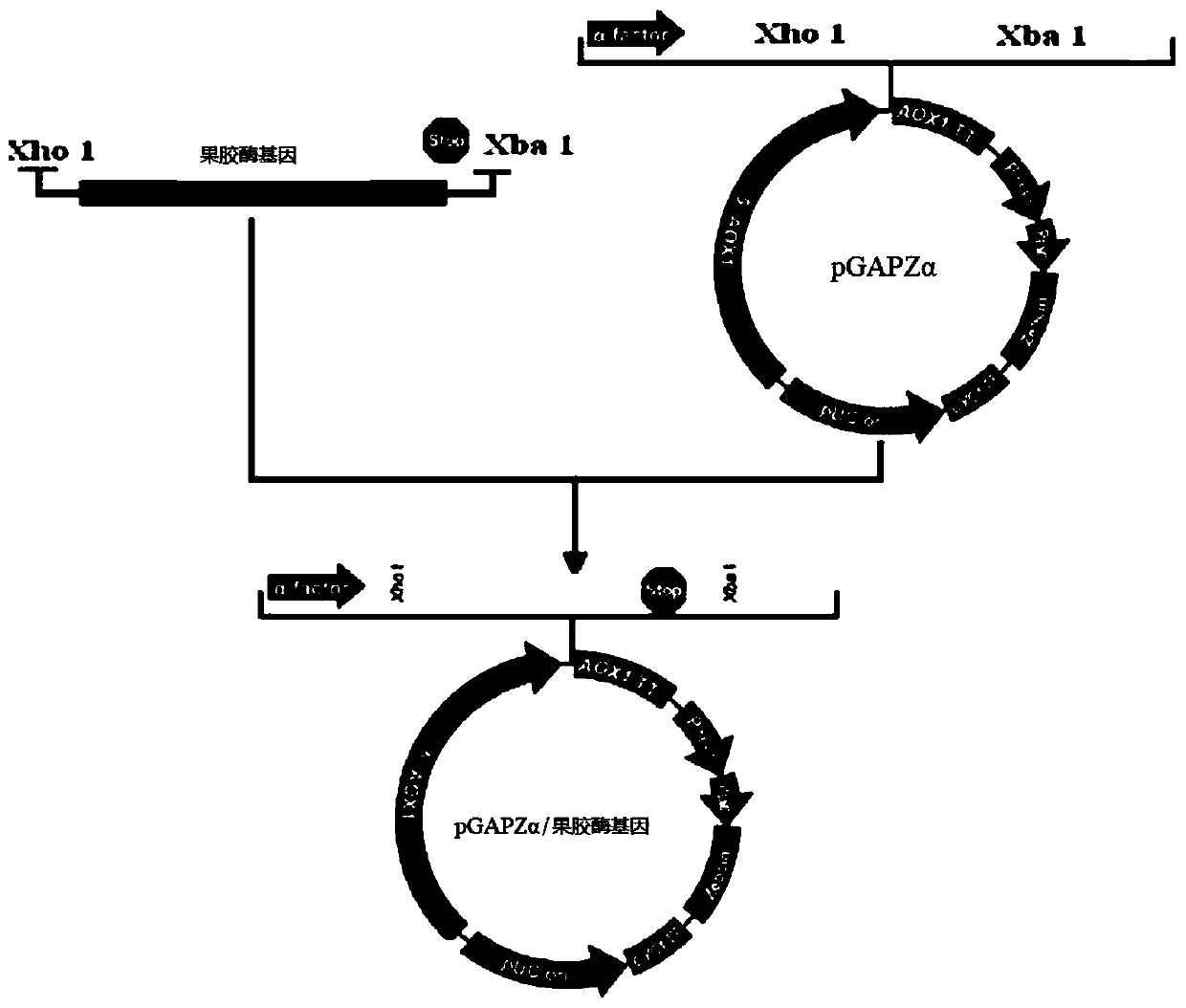
[0049] S1: Construction of expression vector and transformation: The sequence characteristics of the gene itself and the DNA sequence synthesized by yeast codon preference in Example 1, that is, the DNA in SEQ ID No.1, were connected to the constitutive secretory expression vector pGAPZαA of Pichia pastoris to obtain Recombinant vector pGAPZαA-recombinant pectinase, the vector is constructed as figure 1 as shown, figure 1 It is a schematic diagram of the construction of the eukaryotic expression vector pGAPZαA-recombinant pectinase in the embodiment of the present invention. The main vector construction steps are preferably as follows:
[0050] (1) Digest the plasmid containing the synthetic recombinant pectinase gene (SEQ ID No.1) with Xho I and Xba I to obtain the target fragment. The reaction system is as follows (the endonuclease and buffer used were purchas...
Embodiment 3
[0081] In this example, the situation of improving the juice yield of apple, grape and orange juice by the purified recombinant pectinase is tested. The enzyme can increase the yield of fruit juice, the specific steps and results are as follows:
[0082] (1) 0, 1, 2, 4, 8, 16, 32 mg of recombinant pectinase are added to 100 grams of chopped apples respectively, and the apples with different concentrations of recombinant pectinase are fully Beat into pulp and let it stand at room temperature for 60 minutes; pour the apple pulp into 6 layers of gauze respectively, squeeze the juice out of the gauze until no juice flows out, and weigh them separately. The fruit juice weight that weighs is as shown in table 5, as can be seen, the fruit juice weight that the experimental group that has added pectinase obtains is all higher than the fruit juice amount that does not add recombinant pectinase produced, when adding 32 mg of recombinant pectinase When pectinase is added to 100 grams of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com