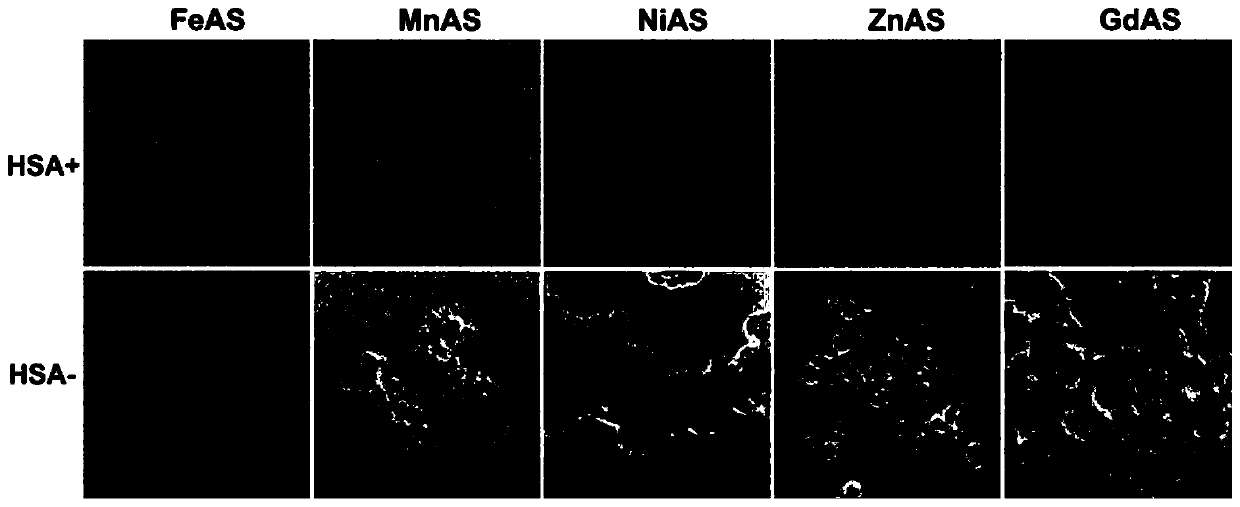
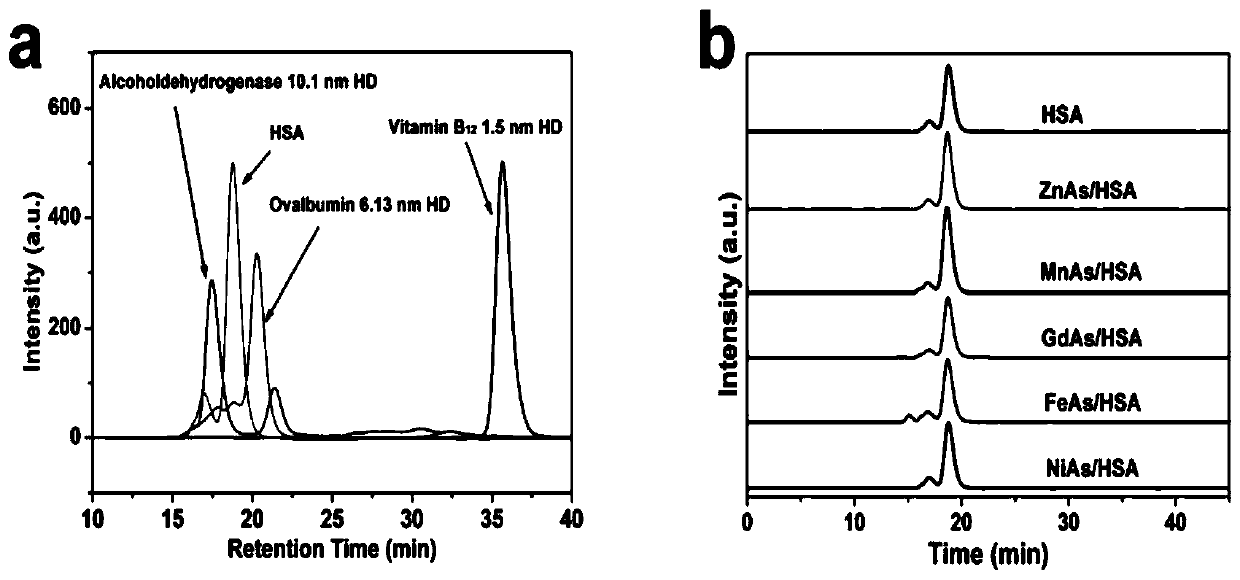
Drug containing arsenic nanoparticles and preparation method thereof
A nanoparticle and drug technology, applied in the field of biomedicine, can solve the problems of not being able to achieve a good therapeutic effect, difficult to degrade and excrete, poor biocompatibility, etc., to achieve clinical transformation potential, and the reaction raw materials are cheap and easy to obtain , the effect of little environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Synthesis of FeAs / HSA
[0042] Dissolve 10 mg of human serum albumin in 20 mL of PBS solution at pH = 3, stir at 37°C and add 40 μL (0.1M) of anhydrous ferric chloride (0.1M) and 40 μL (0.1M) of sodium arsenite dropwise at the same time, add dropwise after 1 hour Ammonia and hydrochloric acid (0.1M) adjust the reaction pH to 5.5-7.4. Continue to react under these conditions for 8 hours and then end the reaction. Centrifuge for 10 minutes to remove the supernatant at a speed of 10,000 r / min. Resuspend the precipitate in pure water and use a 50KD ultrafiltration centrifuge tube to remove unreacted ions and HSA. After washing three times in this way, freeze-dry to obtain FeAs / HSA.
Embodiment 2
[0044] Synthesis of MnAs / HSA
[0045] 10 mg of human serum albumin was dissolved in 20 mL of pure water, 80 μL (0.1 M) of manganese acetate and 40 μL (0.1 M) of sodium arsenite were added dropwise thereto, and the reaction was stirred at 37° C. for 1 hour. After 1 hour, ammonia water or NaOH (1M) was added dropwise to the reaction system to adjust the pH of the reaction solution to 8.0-9.5. After continuing to stir and react at 37°C for 8 hours, centrifuge for 12 minutes to remove the supernatant at a speed of 12000r / min, resuspend the precipitate with pure water and use a 50KD ultrafiltration centrifuge tube to remove unreacted ions and HSA by ultrafiltration. After washing three times in this way, freeze-dry to obtain MnAs / HSA.
Embodiment 3
[0047] Synthesis of NiAs / HSA
[0048] 10 mg of human serum albumin was dissolved in 20 mL of pure water, 80 μL (0.1 M) of nickel acetate and 40 μL (0.1 M) of sodium arsenite were added dropwise thereto, and the reaction was stirred at 37° C. for 1 hour. After 1 hour, aqueous ammonia or NaOH (1M) was added dropwise to the reaction system to adjust the pH of the reaction solution to 7.5-9.5. After continuing to stir and react at 37°C for 8 hours, centrifuge for 10 minutes to remove the supernatant at a speed of 10,000r / min, resuspend the precipitate with pure water and use a 50KD ultrafiltration centrifuge tube to remove unreacted ions and HSA. After washing three times in this way, freeze-dry to obtain NiAs / HSA.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com