Three-dimensional cadmium complex, preparation method thereof, and application of cadmium metal complex as fluorescent probe and photodegradation catalyst
A technology of fluorescent probes and cadmium complexes, applied in organic compound/hydride/coordination complex catalysts, physical/chemical process catalysts, fluorescence/phosphorescence, etc., achieving high yield, good reproducibility, and high yield high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The synthesis of embodiment 1 complex:
[0038] 15.4 mg of cadmium nitrate tetrahydrate (Cd(NO 3 ) 2 4H 2 O), 18.71mg of 1,1'-(4-carboxyphenyl)-2,2'-biimidazole (H 2 L) was dissolved in 10mL water, stirred for half an hour, transferred to the polytetrafluoroethylene liner of a 25mL hydrothermal reactor, and reacted for 72 hours at a temperature of 120°C, and the resulting product was washed three times with ethanol (2mL / time) to obtain Colorless transparent massive crystals. Yield based on metal Cd 52.5%.
Embodiment 2
[0039] The structural characterization of embodiment 2 complex:
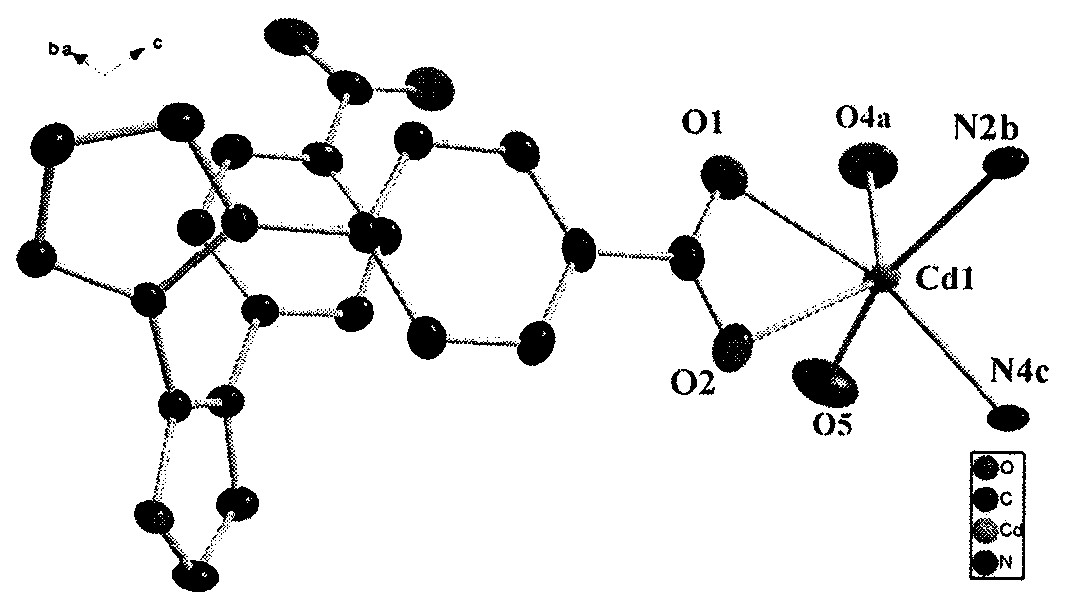
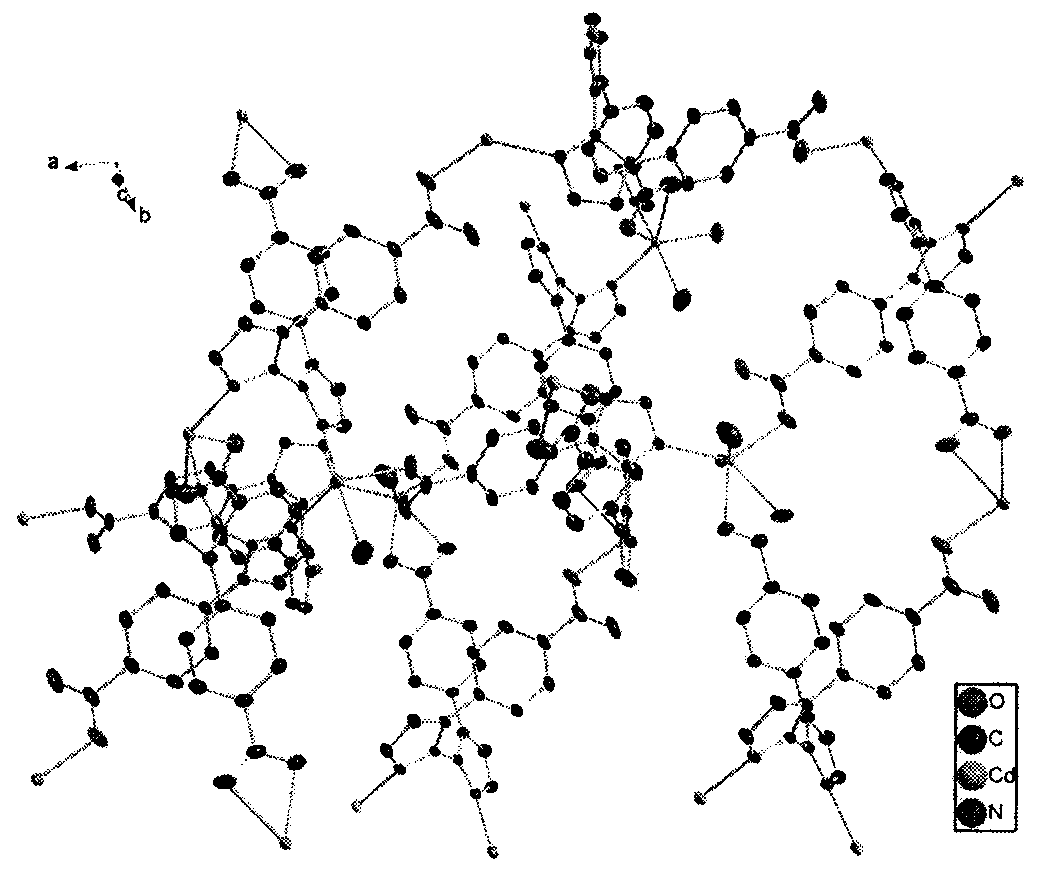
[0040] The crystal structure adopts Bruker Smart CCD X-ray single crystal diffractometer, and at 296(2) K, selects crystals with a size of 0.35×0.15×0.12mm, and uses MoKα rays (λ=0.07107nm) that have been monochromated by graphite as The incident radiation source is used to collect diffraction points by ω / 2θ scanning, the unit cell parameters are refined by the least square method, and the collected data is corrected for absorption with the SADABS program. The structure of the complex was solved by the direct method, the coordinates of non-hydrogen atoms and the anisotropy temperature factor were refined by the full-matrix least-squares method, and all calculations were completed by the SHELXTL program. The detailed crystallographic parameters are listed in Table 1. [Cd(L)(H 2 O)·5H 2 O] n The crystal structure diagram and three-dimensional structure diagram are shown in Fig. 1(a) and Fig. 1(b).
[0041] Cr...
Embodiment 3
[0043] The fluorescent property of embodiment 3 complexes:
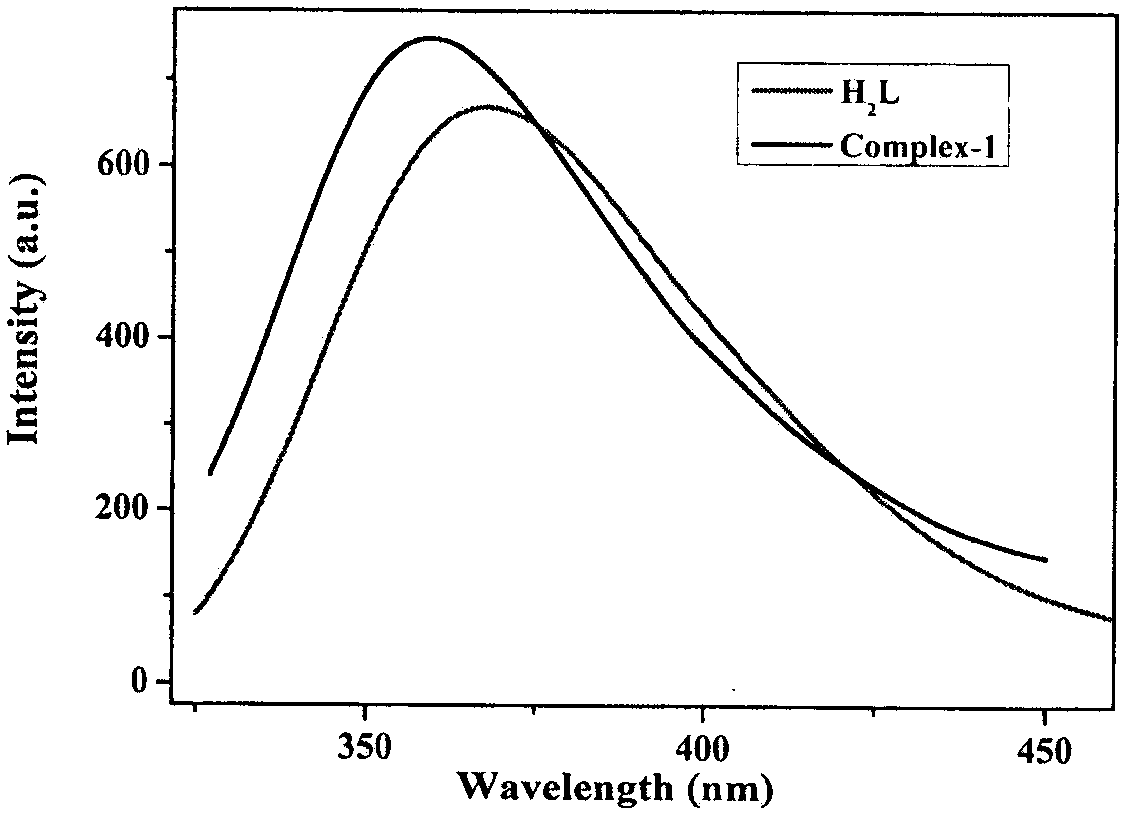
[0044] LS-55 fluorescence spectrometer was used to test the fluorescence properties of the complexes at room temperature. figure 2 It is the solid-state fluorescence image of the cadmium complex prepared in Example 1, indicating that the material has strong fluorescence properties. Such as image 3 As shown, the complex exhibits strong luminescent properties in aqueous solution.
[0045] It can be seen from Figure 4 (a) that in the selected metal ion aqueous solution, the fluorescence intensity of the cadmium complex prepared in Example 1 shows a dependence on the metal ion, and the fluorescence intensity on the Fe 3+ exhibits a complete fluorescence quenching effect and is expected to become a Fe 3+ fluorescent probes.
[0046] It can be seen from Figure 5(a) that in the selected anion aqueous solution, the fluorescence intensity of the cadmium complex prepared in Example 1 shows a dependence on the type of anio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com