Carbon material for heavy metal adsorption as well as preparation method and application thereof
A technology for carbon materials and heavy metals, which is applied in the field of carbon materials adsorbed by heavy metals and its preparation, can solve problems such as large consumption of flocculants, and achieve the effects of reducing adsorption costs and improving adsorption rates.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Add 250mL of concentrated sulfuric acid to 2g of natural flake graphite carbon material. After stirring evenly, add 8g of potassium permanganate in four days. On the fifth day, add 0.5L of ice to the solution to quench it. After cooling, add 30% hydrogen peroxide dropwise to the solution. Turn into bright yellow, centrifuge after stirring for 20 minutes, wash with distilled water-hydrochloric acid-distilled water until the pH remains unchanged.
[0032] The oxidized natural flake graphite carbon material GO was prepared for use.
Embodiment 2
[0034] Take 8.6g of oxidized natural flake graphite carbon material, 4.9g of phosphorous acid and 10mL of phosphorus trichloride, stir, heat and mix, then cool, add 50mL of water after cooling, heat to 65°C and reflux for 48h, collect the solid by suction filtration, add 100mL of water and heat to 75°C Reflux at ℃ for 1h, cool to 45℃ and filter while hot, collect the solid and wash until the pH remains unchanged.
[0035] In the prepared phosphorylated and oxidized natural flake graphite carbon material, the content of phosphorus element is 6.24wt%.
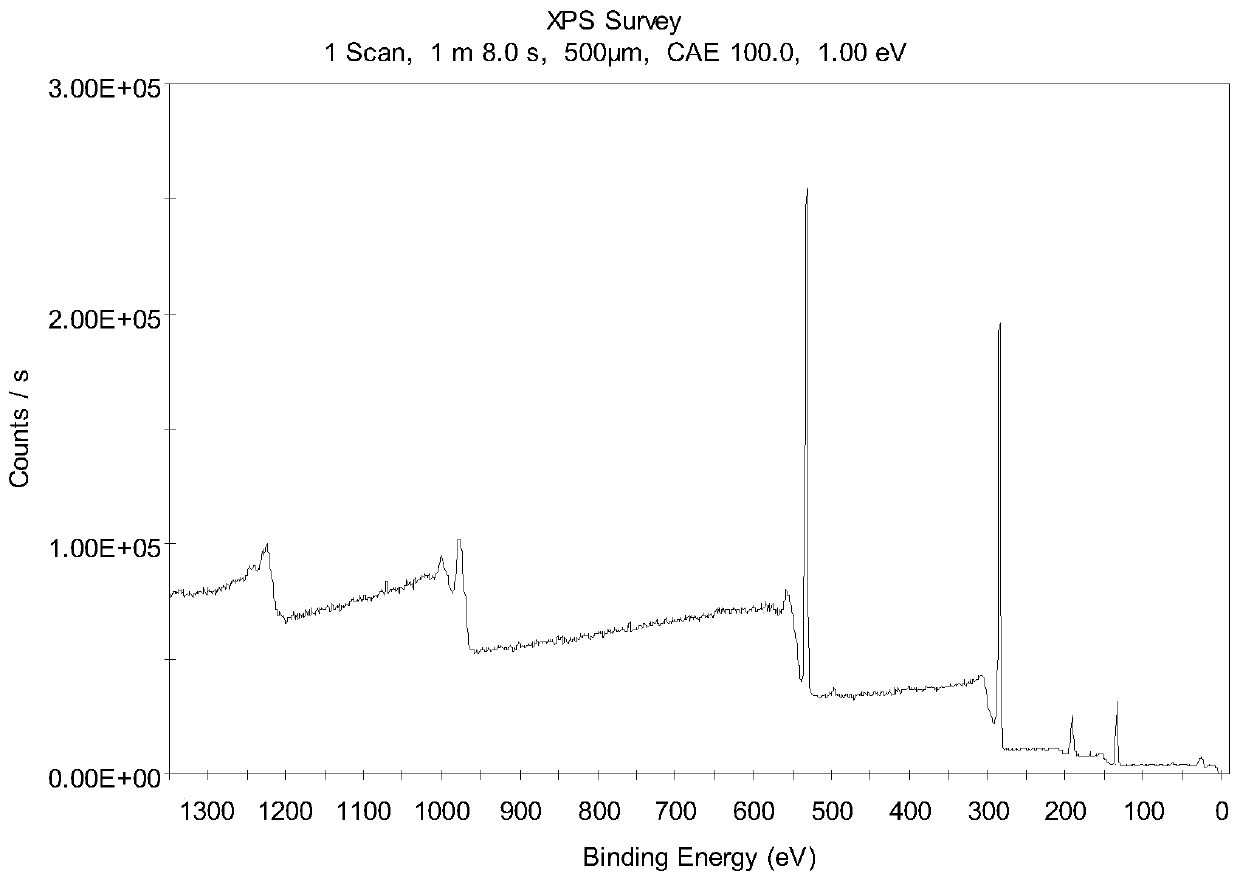
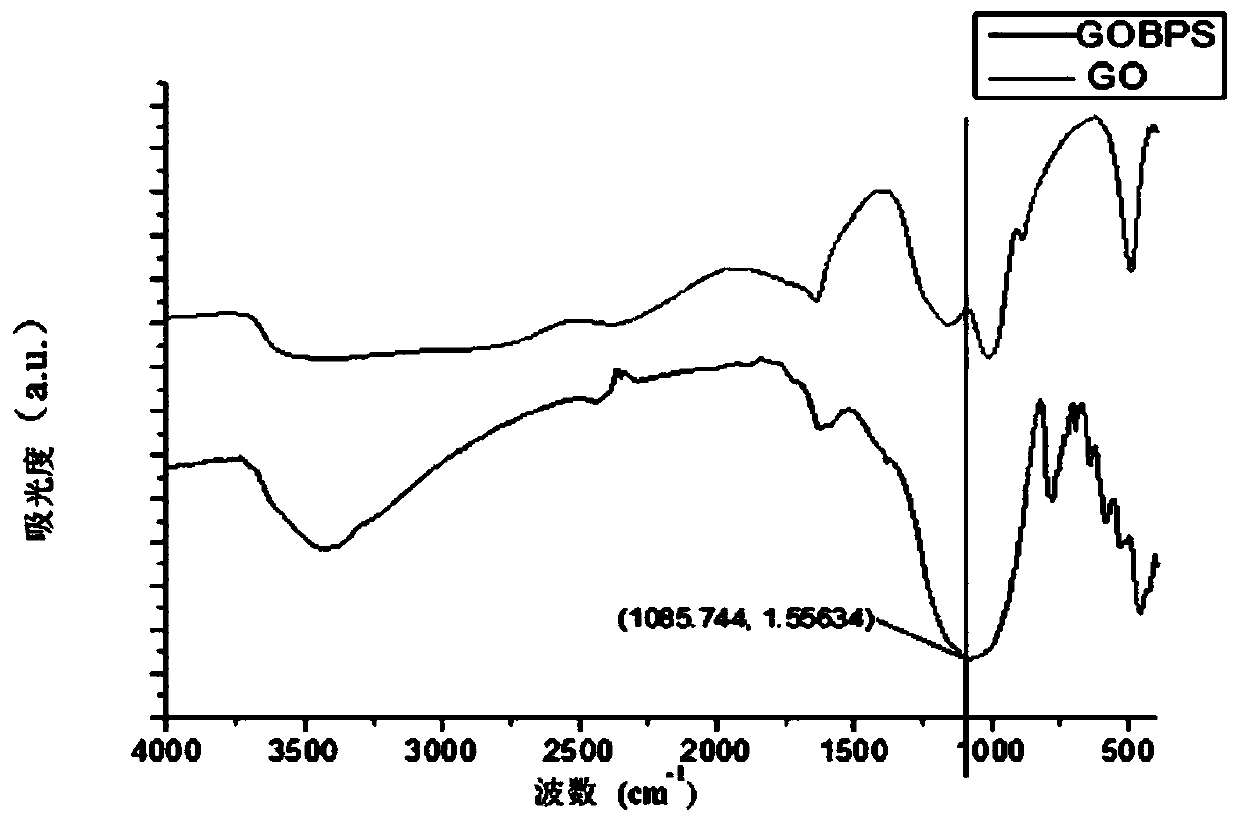
[0036] Characterization of phosphorylated and oxidized natural flake graphitic carbon materials. Fourier transform infrared spectrum shows: at 1085cm -1 The peak of P=O appeared at the position. It shows that there are phosphorus-containing groups on the surface of oxidized natural flake graphite. From the XPS spectrum, there is an obvious 2P peak of phosphorus at the position of 134.9. And the percentage of phosphorus elemen...
Embodiment 3
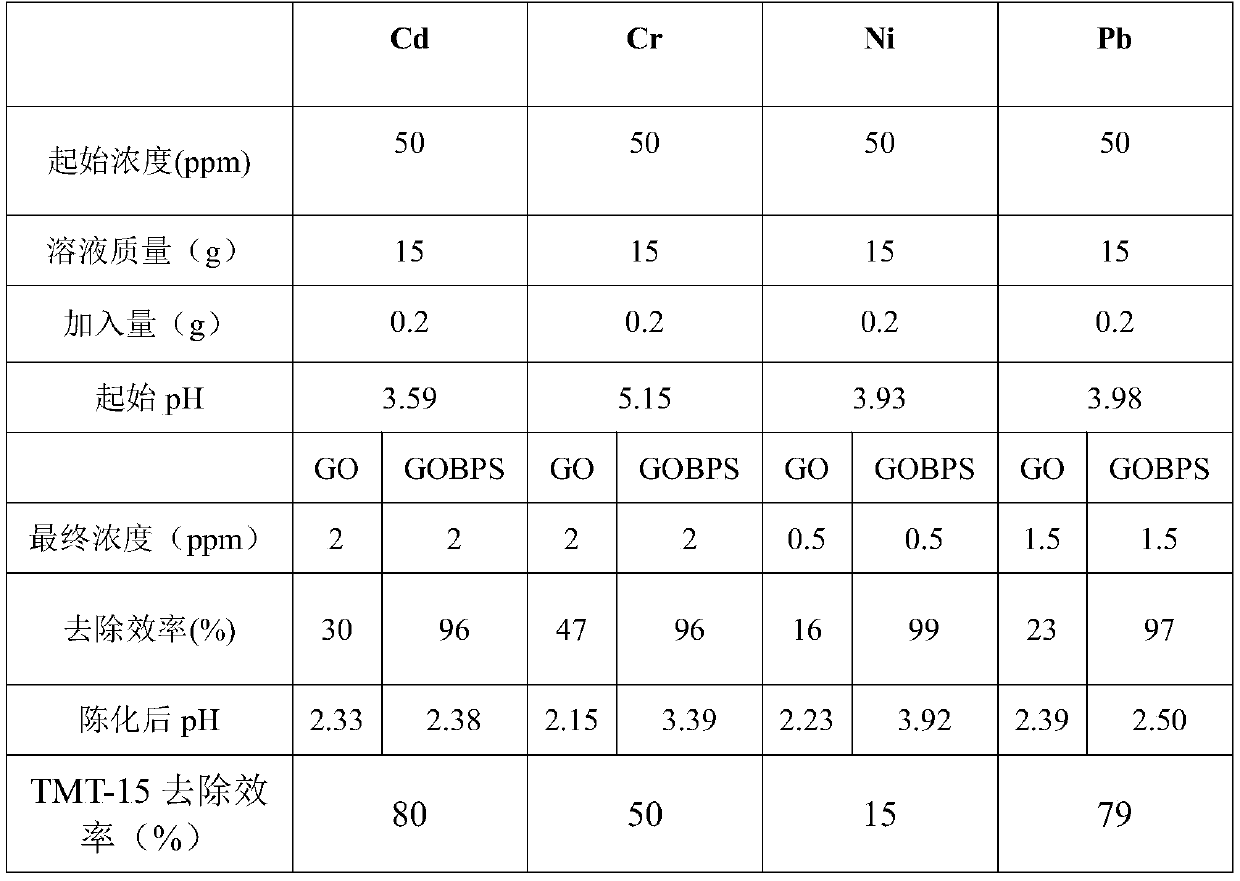
[0038] Take two groups of Cd with a concentration of 50ppm 2+ 、Cr 3+ 、Ni 2+ , Pb 2+ 15g of a single heavy metal ion solution was added with 0.2g of GO and GOBPS respectively, and the GO group was used as a comparison group, and stirred and left for 30 minutes to obtain a treated aqueous solution. The concentration of ions before and after treatment was detected by atomic absorption spectrometry.
[0039] Another set of comparative experiments is TMT-15, a common heavy metal flocculant on the market. Take TMT-152g (high efficiency ratio) and add it to 100g 50ppm single heavy metal ion solution, adjust the pH of the solution at 2-4, and measure the ion concentration after aging. Concentration, and then calculate the removal efficiency.
[0040] In addition, the adsorption efficiency q e (%) is derived according to the following formula:
[0041] q e =(C 0 -Ce) / C 0 ×100%
[0042] Among them, C represents the ion concentration, the unit is mg / L.
[0043] The treated aqu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com