Preparation method and application of Eu<3+>-MOF material
A 3·6H2O, reaction technology, applied in the field of fluorescence sensing, can solve the problems of complex coordination environment and high coordination number of MOF, and achieve high sensitivity, low detection limit and good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: Preparation of iron-based metal organic frameworks.
[0032] This embodiment provides a kind of iron-based MOF material, and its chemical composition is: Fe III (OH)[C 6 h 2 (CO 2 ) 2 (CO 2 h) 2 ]·xH 2 O, its concrete preparation process is as follows:
[0033] Chemically pure or analytically pure FeCl 2 4H 2 O and H 4 BTEC as raw material, according to FeCl 2 4H 2 O: H 4 btec: H 2 O=1:0.5:100 (5mL) (5mL refers to the amount of other substances determined by the amount of 5mL of water, which requires conversion) and weighed in a molar ratio, placed in a polytetrafluoroethylene-lined stainless steel reactor, Stir at room temperature until uniform, seal and place in an oven at 200°C for 48 hours of reaction. After natural cooling, a reddish-brown product is obtained. The obtained product was centrifuged and washed several times with acetone and vacuum-dried at room temperature to obtain an iron-based metal-organic framework.
[0034] The obtaine...
Embodiment 2
[0035] Example 2: Eu 3+ - Preparation of MOF materials.
[0036] This example provides a post-utilization preparation method for obtaining Eu 3+ -MOF material, its specific preparation process is as follows:
[0037] By mixing 0.1g Fe-based MOF material and 0.0892g Eu(NO 3 ) 3 ·6H 2 A mixture of O was stirred in 10 mL of aqueous solution for 24 hours to prepare Eu 3+ -MOF materials. The resulting brown solid was then separated from the mixed dispersion by centrifugation and washed with an aqueous solution to remove excess Eu 3+ , and the obtained brown powder was vacuum-dried at 60 °C for 8 h.
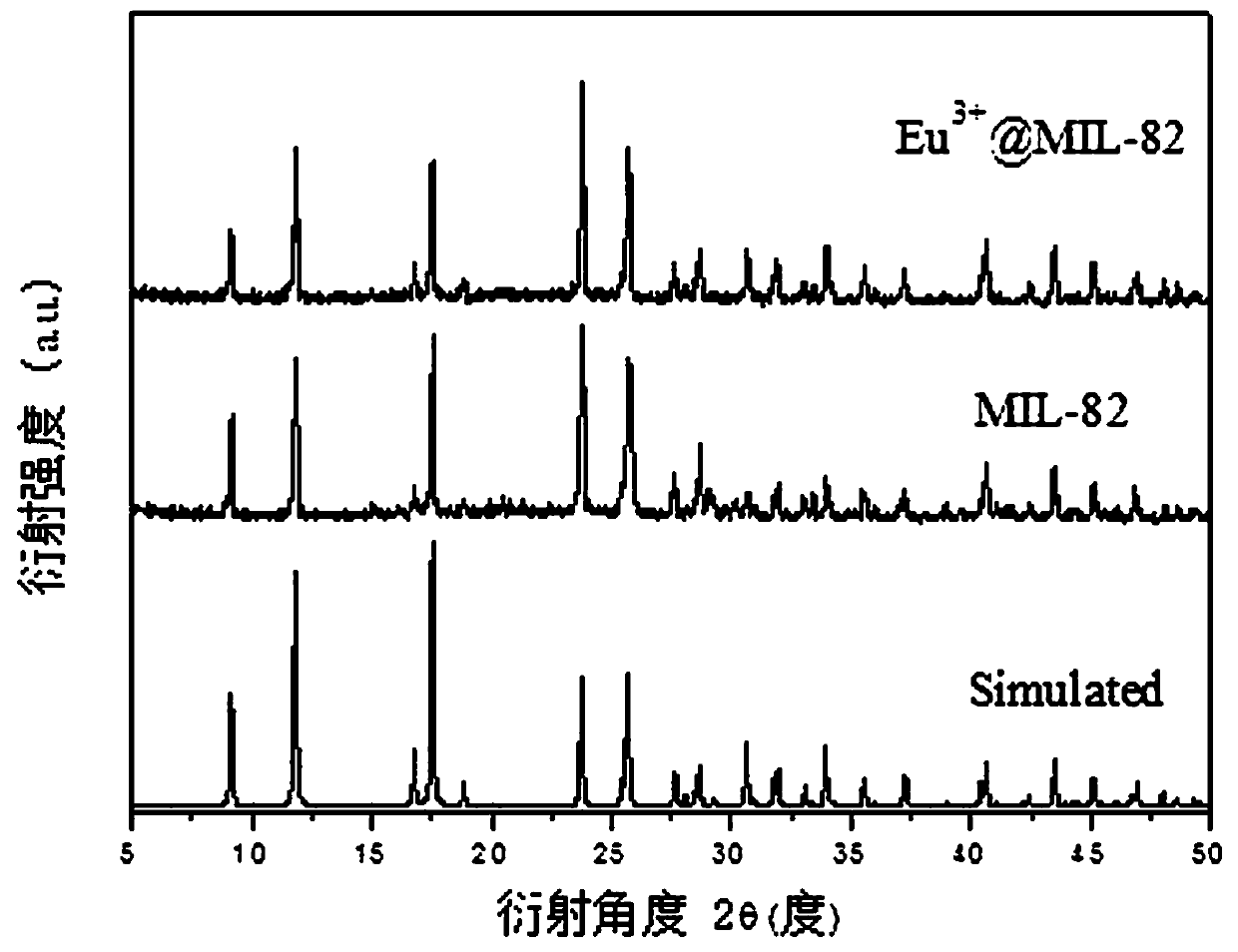
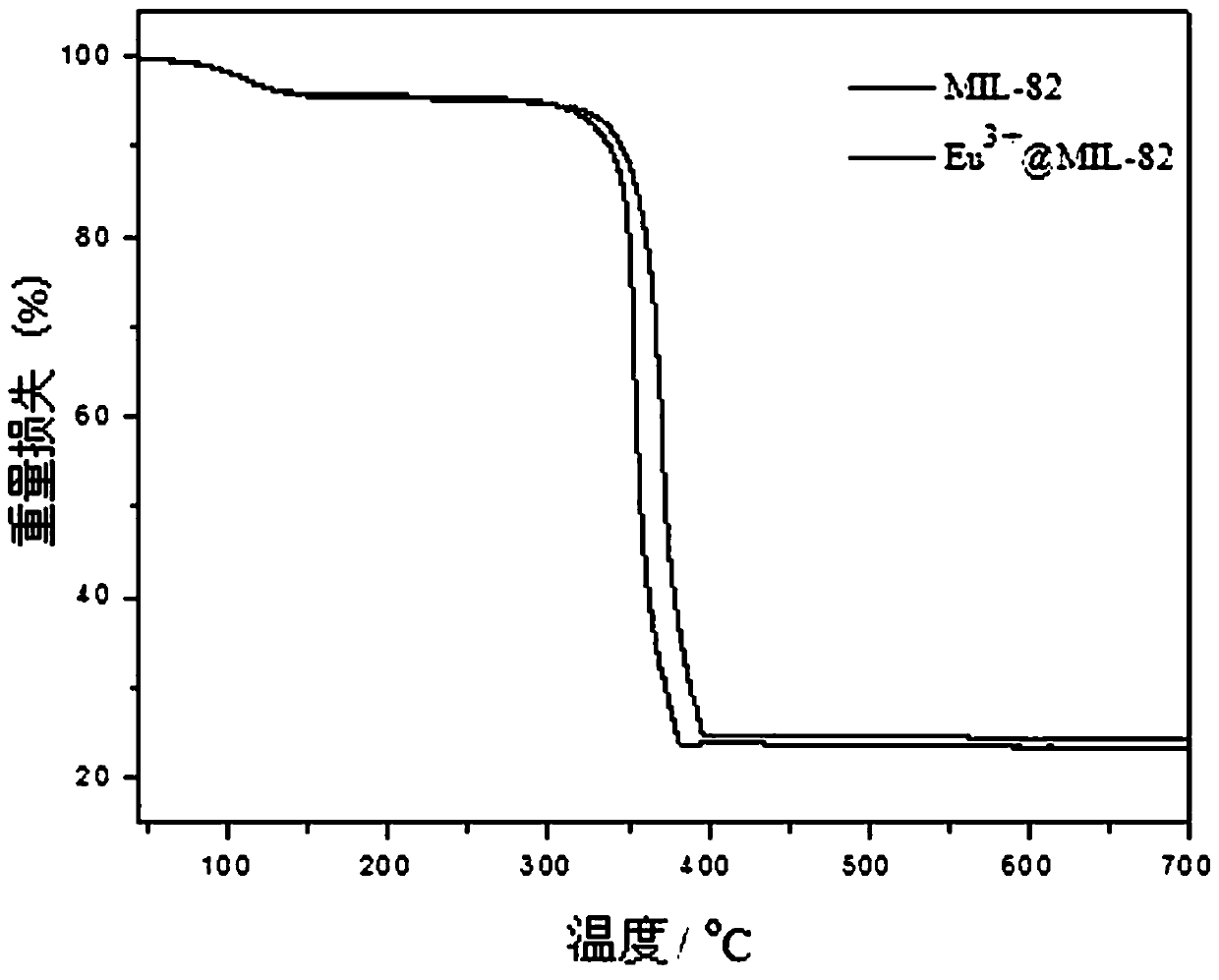
[0038] For the obtained Eu 3+ -MOF materials were characterized by powder X-ray (PXRD) pattern and thermogravimetric (TG) analysis. figure 1 The PXRD pattern of the obtained sample is given, compared with the crystal structure pattern of the iron-based MOF material, and the synthesized Eu 3+ -MOF materials did not change in crystal structure, indicating that Eu 3+ Doping doe...
Embodiment 3
[0039] Embodiment 3: Fluorescence sensing experiment.
[0040] This embodiment uses the Eu prepared in Example 2 3+ -MOF material for fluorescence sensing experiments, the specific experimental process is as follows:
[0041] The powder prepared by 3mg embodiment 2 was dispersed in M(NO 3 ) x Aqueous solution (3ml, 1×10 -2 mol / L) (M n+ =Ag + , Pb 2+ , Ca 2+ , Zn 2+ , Hg 2+ , Ni 2+ , Mg 2+ ,Co 2+ , Cd 2+ , Na + , Cr 3+ , Al 3+ , Fe 3+ ). The mixture was then sonicated for 10 min to form a suspension doped with metal ions for luminescence measurements. All luminescence data were recorded at room temperature.
[0042] The samples obtained in Example 2 were tested for the emission spectrum. Luminescence spectra of mixed suspensions doped with metal ions were recorded at room temperature. Figure 4 As shown, the visible material exhibits Eu 3+ strong characteristic emission peaks. Figure 5 The photoluminescence spectra of mixed suspensions of various metal i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com