A kind of donor material for indacene and difuran-based organic solar cells, its preparation method and application
A solar cell and furan-based technology, which is applied in the field of Indanodifuran-based organic solar cell donor materials, can solve the large-scale preparation and promotion of unfavorable polymer materials, increase the degree of polymer conjugation, and increase the preparation of polymers Cost and other issues, to achieve the effect of good photophysical properties and photovoltaic performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
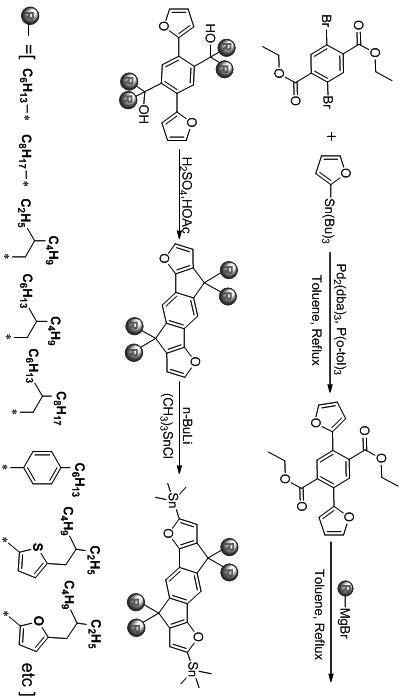
[0047] Synthesis of Indacenedifuran (IDF)-based Donor Material 6a for Organic Solar Cells:
[0048]
[0049] (1) Preparation of compound 2
[0050] Diethyl 2,5-dibromo-terephthalate (compound 1, 2.0 g, 5.26 mmol), 2-tributyltinfuran (3.36 g, 10.68 mmol), tris(dibenzylideneacetone) dipalladium ( 0.46 g, 0.05 mmol), tri(o-tolyl)phosphine (0.03 g, 0.1 mmol) were placed in a 250 mL single-necked flask, vacuumed and filled with nitrogen for 3-4 times, and anhydrous toluene was added under nitrogen protection for 100 mL. Place the reaction flask in an oil bath and heat to 115 o C overnight (12~18h). After the mixture was cooled to room temperature, the mixed liquid was poured into deionized water, and the aqueous phase was extracted with 3×30 mL of dichloromethane. Combine the organic phases, wash with saturated aqueous sodium chloride, collect the organic phases and dry over anhydrous magnesium sulfate, filter, and spin out the solvent under reduced pressure. Separation by ...
Embodiment 2
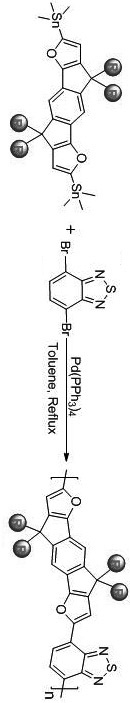
[0066] Preparation of Compound PIDFC8
[0067] The product structural formula is as follows:
[0068]
[0069] The indacene difuran unit IDFC8-T is the same as IDFC2C4-T, except that n-octylmagnesium bromide is replaced by isooctylmagnesium bromide in step (2). The corresponding polymer synthesis route is IDFC8-T (0.18 mmol, 181.6 mg), 4,7-dibromo-2,1,3-benzothiadiazole (0.2 mmol, 60.3 mg), tetrakis (triphenylphosphine ) palladium (10.0 mg, 0.0087mmol) 2 25.0 mL single-necked flask with protection device. Then, vacuumize and inflate with nitrogen for 3-4 times, and inject 9 mL of anhydrous toluene and 0.1 mL of anhydrous N,N'-dimethylformamide (base catalyst) with a syringe under nitrogen protection. After the addition was complete, the mixture was evacuated again and filled with nitrogen. Then quickly heat up to 115 o C, and observe the change in the viscosity of the mixture. When the rotation speed of the rotor in the reaction bottle becomes slow and the mixture bec...
Embodiment 3
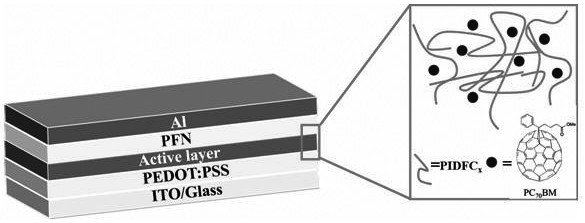
[0072] The indaprodifuryl organic solar cell donor material (PIDFC2C4 and PIDFC8) synthesized in the example 1-2 is configured into 10 with chlorobenzene -4mg / mL solution, and use a pipette gun to measure 50 μL of chlorobenzene solution and spin-coat it on pretreated quartz glass (15mm×15mm), let it dry naturally, and test the ultraviolet-visible absorption spectrum of the polymer film, such as Figure 4 shown. According to the UV-visible absorption spectrum of the film through the formula E gap =1240 / λ onset The calculated optical energy gaps of PIDFC2C4 and PIDFC8 under the spectrum are 1.68eV and 1.61eV respectively, which belong to narrow bandgap polymer donor materials.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com