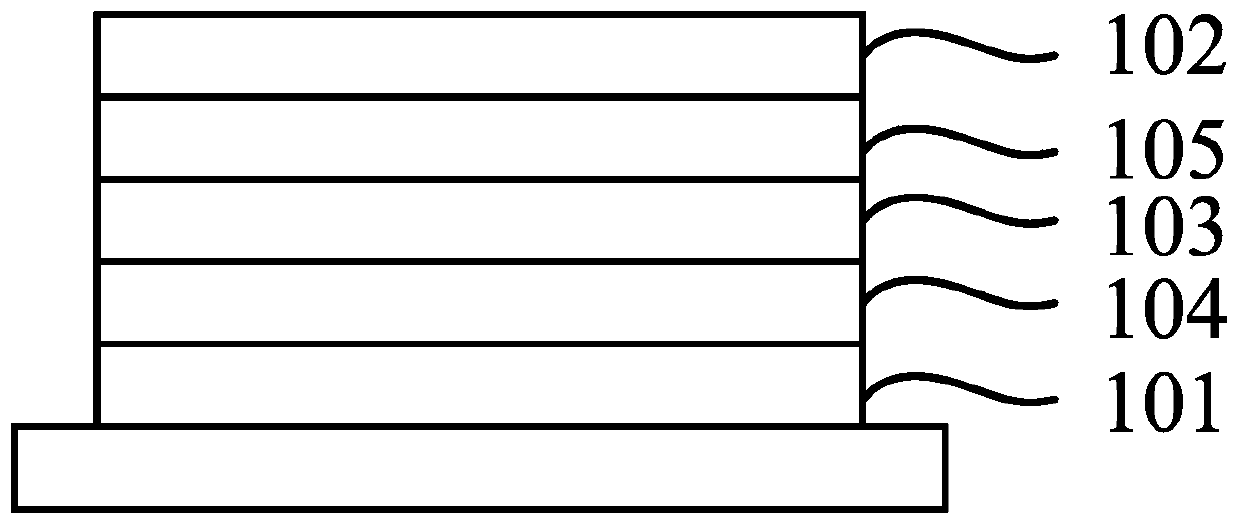
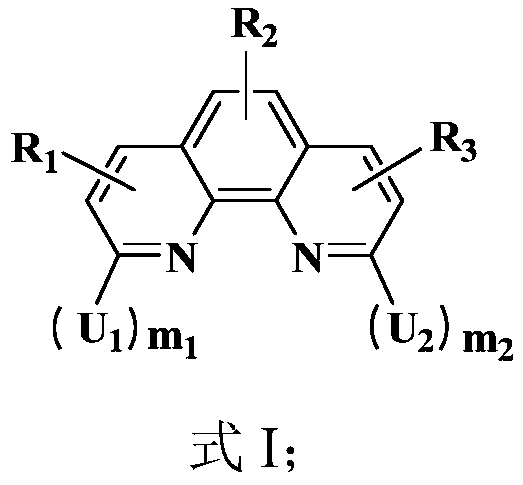
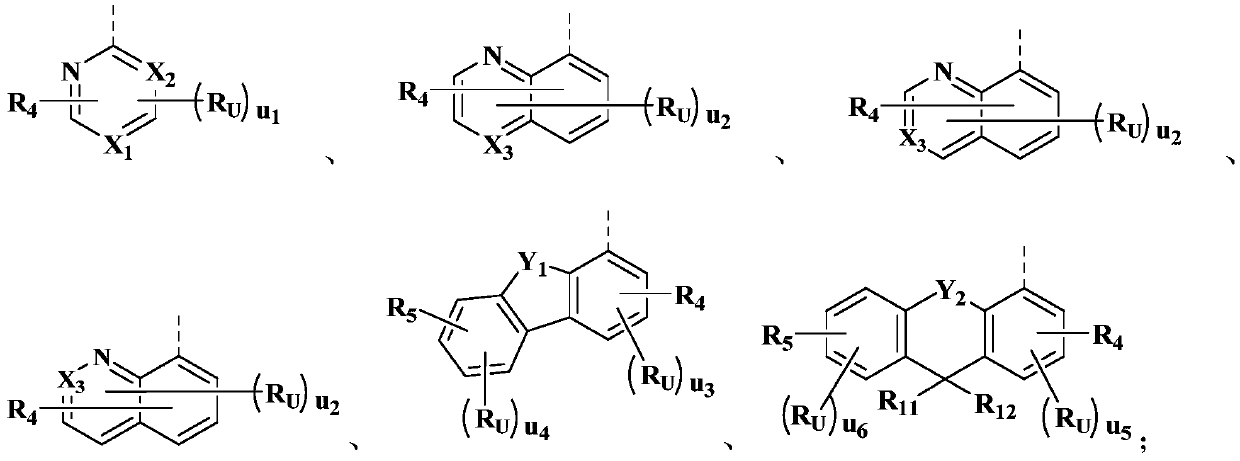
Organic compound, and electron transport material and applications thereof
An organic compound, selected technology, applied in the field of organic compounds and electron transport materials, can solve problems such as low electron mobility, unbalanced electron transport and hole transport, and failure to meet the performance requirements of electroluminescent devices, and achieve the purpose of inhibiting molecular The increase of the gravitational force, the improvement of luminous efficiency and working life, and the effect of improving the drift voltage rise
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0131] This embodiment provides an organic compound with the following structure:
[0132]
[0133] The preparation method of the organic compound M16 comprises the following steps:
[0134] (1)
[0135] In a 100mL three-neck flask, first dissolve S1 (10mmol, wherein TMS is trimethylsilyl) in 20mL of concentrated sulfuric acid, and use an ice-water bath to control the temperature; after stirring evenly at a certain speed, add concentrated nitric acid to the reaction system (15mmol) to react for 2h; under ice-water bath, adjust the reaction solution to neutral with NaOH aqueous solution; after the reaction, extract with ether, dry the obtained organic phase with anhydrous sodium sulfate, distill off the solvent, and use column Purified by analytical method to obtain intermediate S2 (9.5 mmol, yield 95%).
[0136] Test the structure of intermediate S2: MALDI-TOFMS (m / z) obtained by matrix-assisted laser desorption ionization time-of-flight mass spectrometry: C 12 h 13 C...
Embodiment 2
[0158] This embodiment provides an organic compound with the following structure:
[0159]
[0160] The preparation method of the organic compound M19 comprises the following steps:
[0161] Obtain intermediate S10 according to the method of steps (1) to (6) in Example 1;
[0162] (7)
[0163] Intermediate S10 (1mmol) and tetramethylethylenediamine (2.5mmol) were added to 90mL THF, the temperature was lowered to -78°C in an ethanol bath, and tert-butyllithium (t-BuLi, 2.5mmol) was added within 15min Dropwise into the reaction system, stirred at low temperature for 1h. Add S12 (2.5 mmol) at low temperature, stir at low temperature for 1 h, then return to room temperature naturally, and stir overnight. After the reaction, extract with water and ethyl acetate, the organic phase was washed with anhydrous Na 2 SO 4 dry. The crude product was purified by silica gel column chromatography, using a mixed solvent of n-hexane and chloroform at a volume ratio of 5:1 as the elue...
Embodiment 3
[0168] This embodiment provides an organic compound with the following structure:
[0169]
[0170] The preparation method of the organic compound M2 comprises the following steps:
[0171] Obtain intermediate S10 according to the method of steps (1) to (6) in Example 1;
[0172] (7)
[0173] Dissolve intermediates S10 (2mmol) and S13 (1mmol) in 30mL toluene under nitrogen protection, add Pd(PPh 3 ) 4 (0.1mmol) as a catalyst, add 4mL potassium carbonate aqueous solution (2mol / L), and reflux for 12h. After the reaction, it was extracted three times with saturated brine and ethyl acetate, and the organic phases were combined and dried over anhydrous sodium sulfate. All the solvent was distilled off under reduced pressure, and the crude product was collected. The crude product was purified by silica gel column chromatography, using a mixed solvent of n-hexane and chloroform at a volume ratio of 5:2 as the eluent, and finally purified to obtain the solid target product M...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com