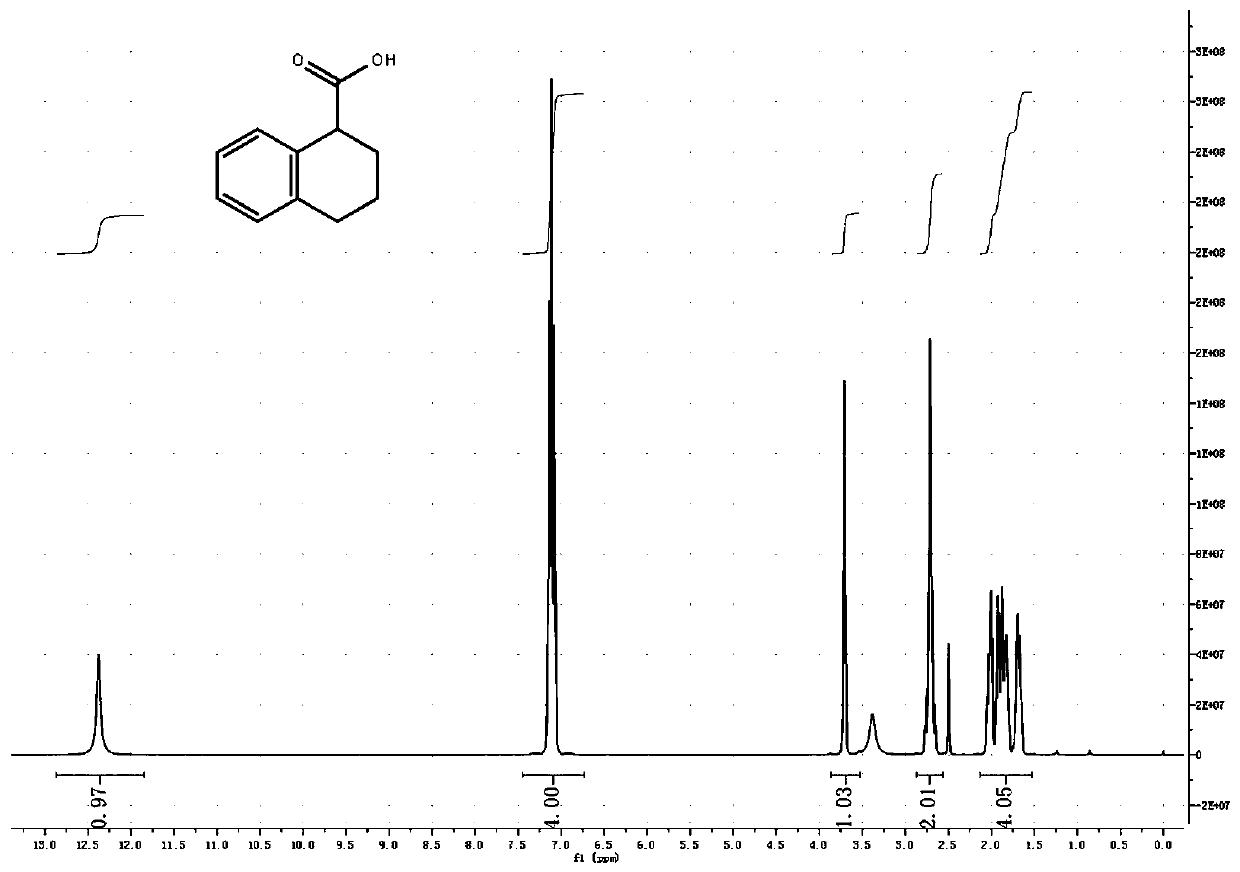
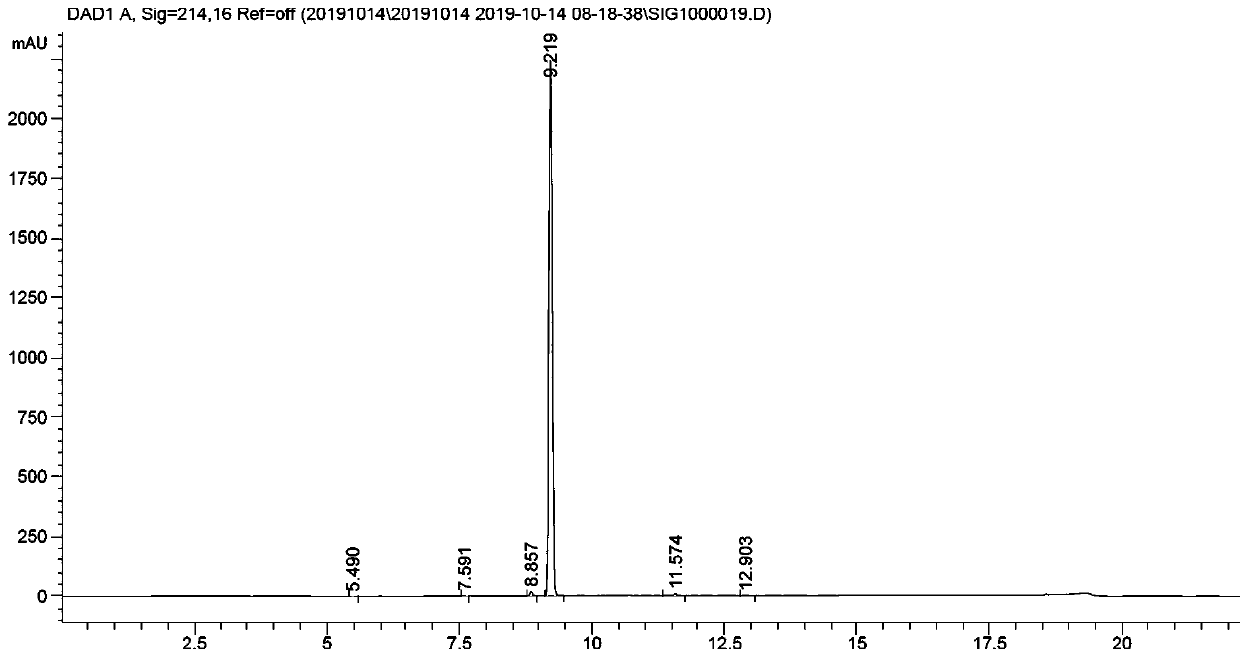
Method for preparing 1,2,3,4-tetrahydro-1-naphthoic acid by using superstrong alkali method
A super base and naphthoic acid technology, which is applied in the preparation of carboxylate, carboxylate, and carbon-based compounds, etc., can solve the problems of long route, non-reaction, and low yield, and achieve high operability, Convenient for industrialization and good repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] In a 1000ML three-neck flask, equipped with a thermometer and a nitrogen-protected mouthpiece, put 19.8g of tetrahydronaphthalene under nitrogen protection, add 200ml of n-hexane, add 59.6g of potassium tert-butoxide, control the temperature at 0°C, and add dropwise 2.5M n-butyllithium 72ml, release heat, control the temperature below 10°C, after dripping, react at 10-20°C for 1 hour, set aside.
[0058] The temperature of the system was lowered to -70°C, and dry carbon dioxide was introduced for 30 minutes. The system released heat, and the temperature was raised to -30°C. The temperature was controlled at -30 to -70°C, and the reaction was carried out for 1 hour. Followed by HPLC, until the residual material remained below 10%.
[0059] Cool down to -70°C, extract the reaction solution with 25% dilute sulfuric acid until the pH is less than 1, raise it to room temperature, stir for 30 minutes, and the extraction is complete.
[0060] Then use 50% liquid caustic soda t...
Embodiment 2
[0062] In a 500ML three-necked flask, equipped with a thermometer and a nitrogen protection mouthpiece, put 19.8g tetralin under nitrogen protection, add 200ml n-hexane, add 59.6g potassium tert-butoxide, control the temperature at 0°C, and add dropwise 2.5M n-butyllithium 72ml, release heat, control the temperature below 10°C, after dripping, react at 10-20°C for 1 hour, set aside.
[0063] A 1000ml three-neck flask was equipped with a thermometer and a nitrogen-protected mouthpiece. Under nitrogen protection, 100g of dry ice was added, and the spare solution was added dropwise to the system, stirred and reacted for 30 minutes, followed by HPLC until the remaining raw materials were less than 5%.
[0064] Cool down to -70°C, extract the reaction solution with 25% dilute sulfuric acid until the pH is less than 1, raise it to room temperature, stir for 30 minutes, and the extraction is complete.
[0065] Then use 50% liquid caustic soda to adjust the pH to be greater than 11, s...
Embodiment 3
[0067] In a 50L reaction kettle, equipped with a thermometer and a mouthpiece protected by nitrogen gas, put 3000g of tetralin under nitrogen protection, add 10kg of n-hexane, add 9.05kg of potassium tert-butoxide, control the temperature at 0°C, and add dropwise 2.5M n-butyllithium 10.9 L, exothermic, control the temperature below 10°C, after dropping, react at 10-20°C for 1 hour, set aside.
[0068] In a 100L reaction kettle, equipped with a thermometer and a mouthpiece protected by nitrogen gas, under nitrogen protection, add 20Kg of dry ice, press the spare liquid into the dry ice system with nitrogen, start without stirring, wait until the temperature of the system rises to -50°C, start stirring, and react 60 minutes, HPLC tracking, until the raw material remains below 5%.
[0069] The reaction solution was extracted with 10 liters of water, raised to room temperature, stirred for 30 minutes, and the extraction was completed.
[0070] Layering, standing for layering, dis...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com