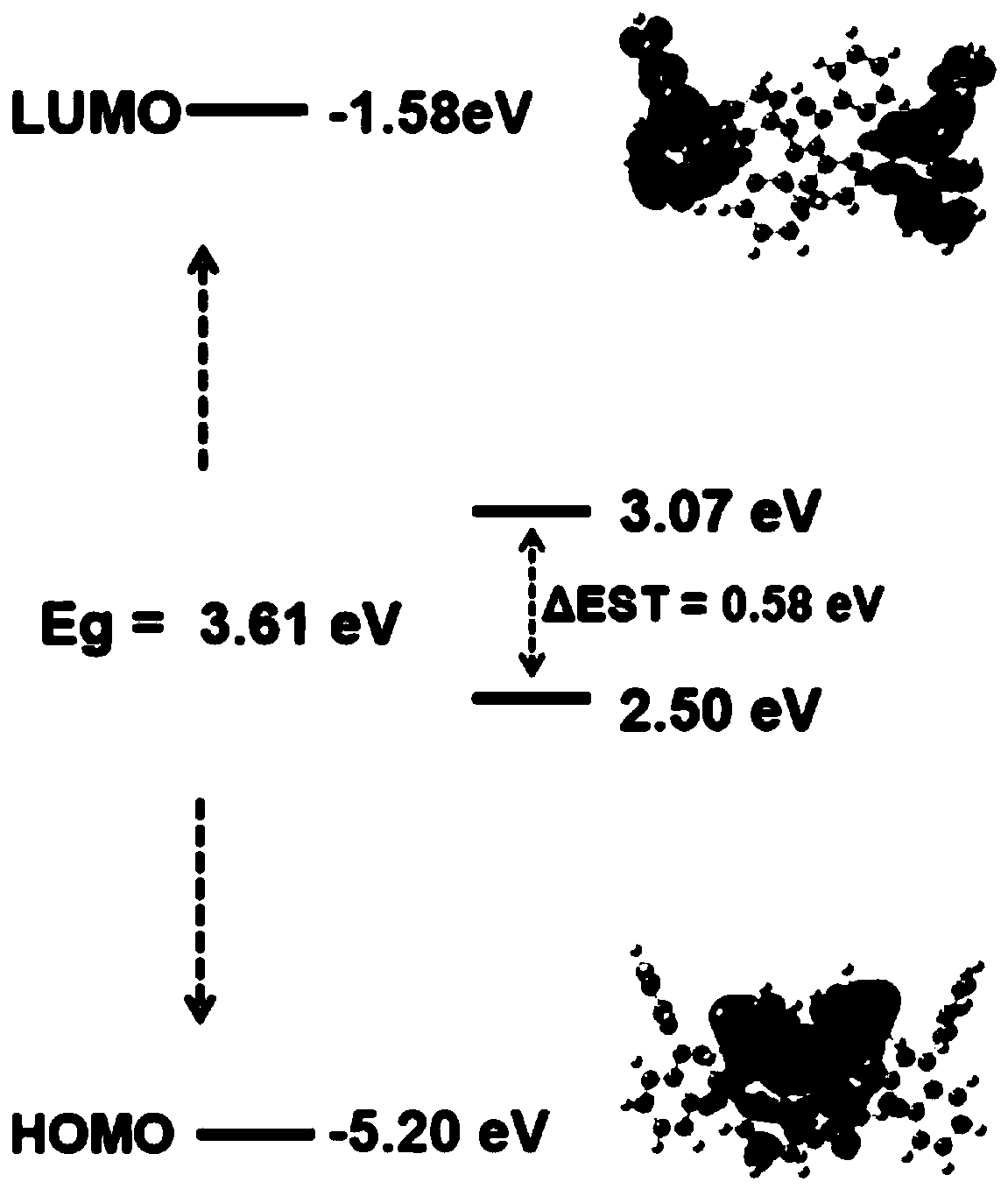
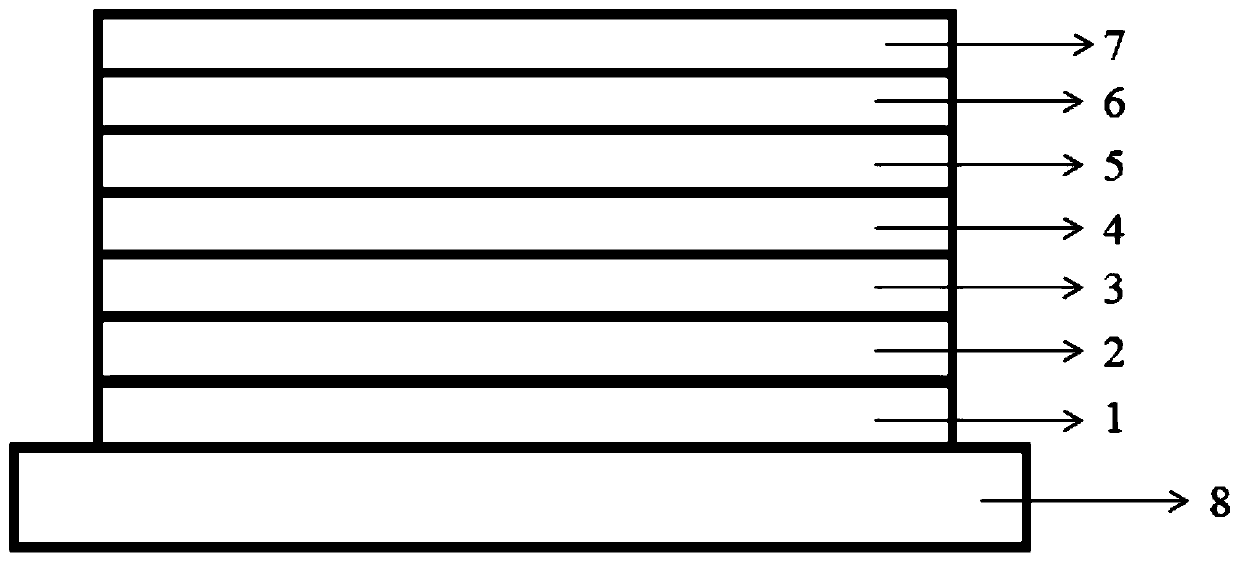
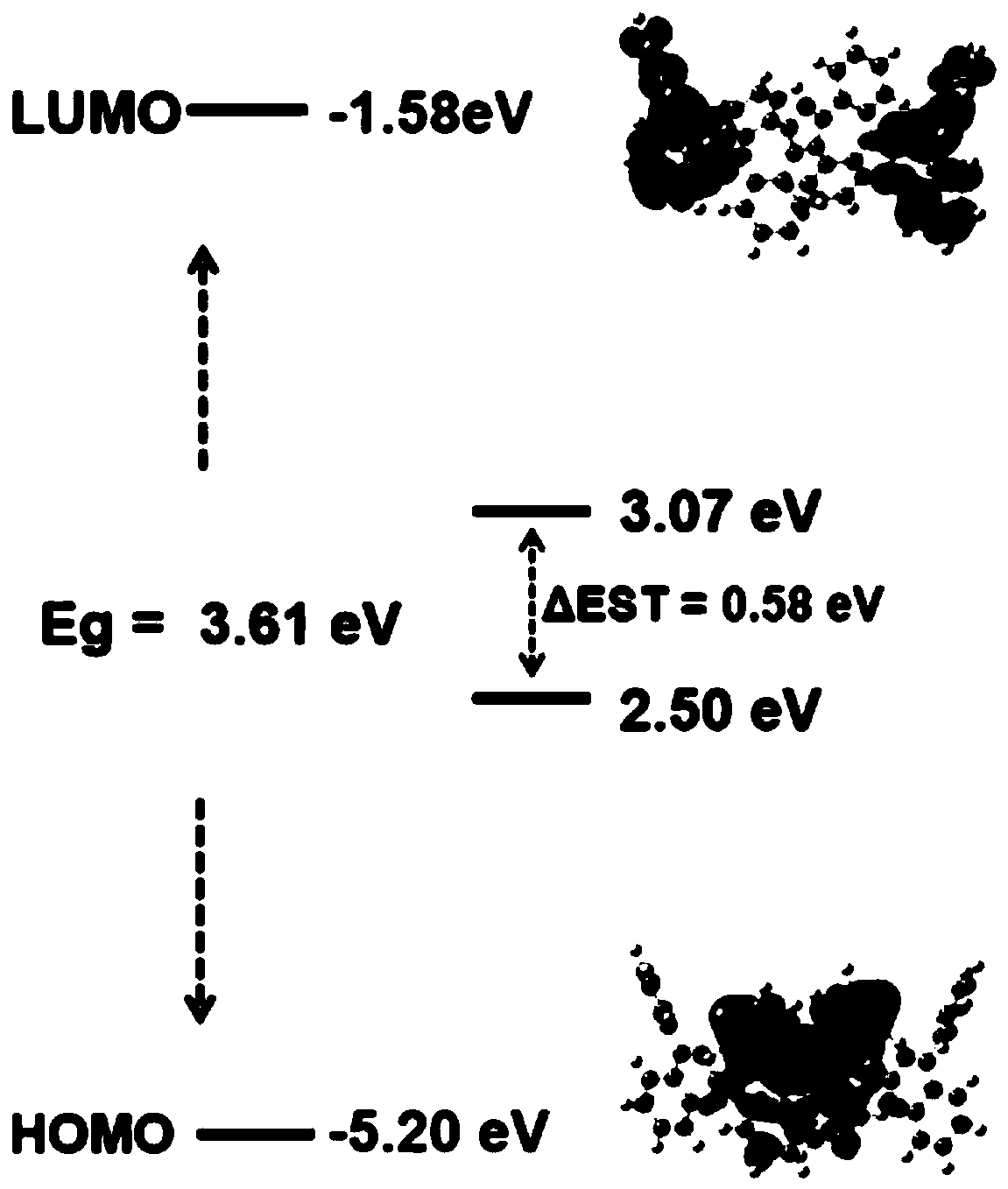
Nitrogen heterocyclic compound and preparation method thereof, organic electroluminescent material containing nitrogen heterocyclic compound, luminescent layer and application of luminescent layer
A nitrogen heterocyclic compound and compound technology, applied in luminescent materials, chemical instruments and methods, organic chemistry, etc., can solve the problem of OLED device performance and luminous efficiency decrease, affecting OLED device luminous efficiency, electron and hole transport imbalance and other problems, to achieve excellent hole transport performance and electron transport performance, improve performance and luminous efficiency, thermal stability and morphological stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0055] This preparation example provides a nitrogen heterocyclic compound C1, which has the following structure:
[0056]
[0057] The preparation method is as follows:
[0058]
[0059] (1) Synthesis of intermediate A1:
[0060] Under nitrogen protection, add tetrabromothiophene (400g, 1.0mol), phenylboronic acid (254g, 2.1mol), potassium carbonate (276g, 2.0mol), toluene 2800mL, ethanol 1200mL into a 10.0L three-neck flask stirred by a thermometer. Mix with 1200mL of water, heat up to 60°C, add catalyst tetrakis(triphenylphosphine)palladium 2.31g (0.002mol), react at 70°C for 6 hours, cool down to room temperature, extract with dichloromethane, wash with water three times, anhydrous sodium sulfate Drying, rotary evaporation to remove most of the solvent, adding n-hexane for recrystallization, and drying to obtain 335g of the main ligand A1 (yield: 85%);
[0061] (2) Synthesis of intermediate B1:
[0062] Under nitrogen protection, in a 5L three-necked flask, add int...
preparation example 2
[0070] This preparation example provides a nitrogen heterocyclic compound C2, which has the following structure:
[0071]
[0072] The preparation method is as follows:
[0073] The difference from Preparation Example 1 is that step (4) is different, and bromobenzene is replaced by 2-bromonaphthalene (the molar weight is unchanged), and the yield is 81%.
[0074] Elemental analysis: (C 48 h 30 N 2 S) Theoretical value: C, 86.46; H, 4.53; N, 4.20; S, 4.81; Measured value: C, 86.47; H, 4.54; N, 4.22; S, 4.83;
[0075] HRMS(ESI)m / z(M + ): theoretical value: 666.84; measured value: 666.90.
preparation example 3
[0077] This preparation example provides a nitrogen heterocyclic compound C3, which has the following structure:
[0078]
[0079] The preparation method is as follows:
[0080] The difference from Preparation Example 1 is that the step (4) is different, and the bromobenzene is replaced by 4-bromobiphenyl (the molar weight is unchanged), and the yield is 83%.
[0081] Elemental analysis: (C 52 h 34 N 2 S) Theoretical value: C, 86.88; H, 4.77; N, 3.90; S, 4.46; Measured value: C, 86.89; H, 4.78; N, 3.92; S, 4.45;
[0082] HRMS(ESI)m / z(M + ): theoretical value: 718.92; measured value: 718.95.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com