Method for preparing ammonium sulfate and sodium bicarbonate from sodium sulfate
A technology of sodium bicarbonate and sodium bicarbonate mother, applied in the field of chemical technology, can solve problems such as difficult recycling, increased operating costs, and complicated processes, and achieves the effects of easy industrial process, improved utilization rate, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
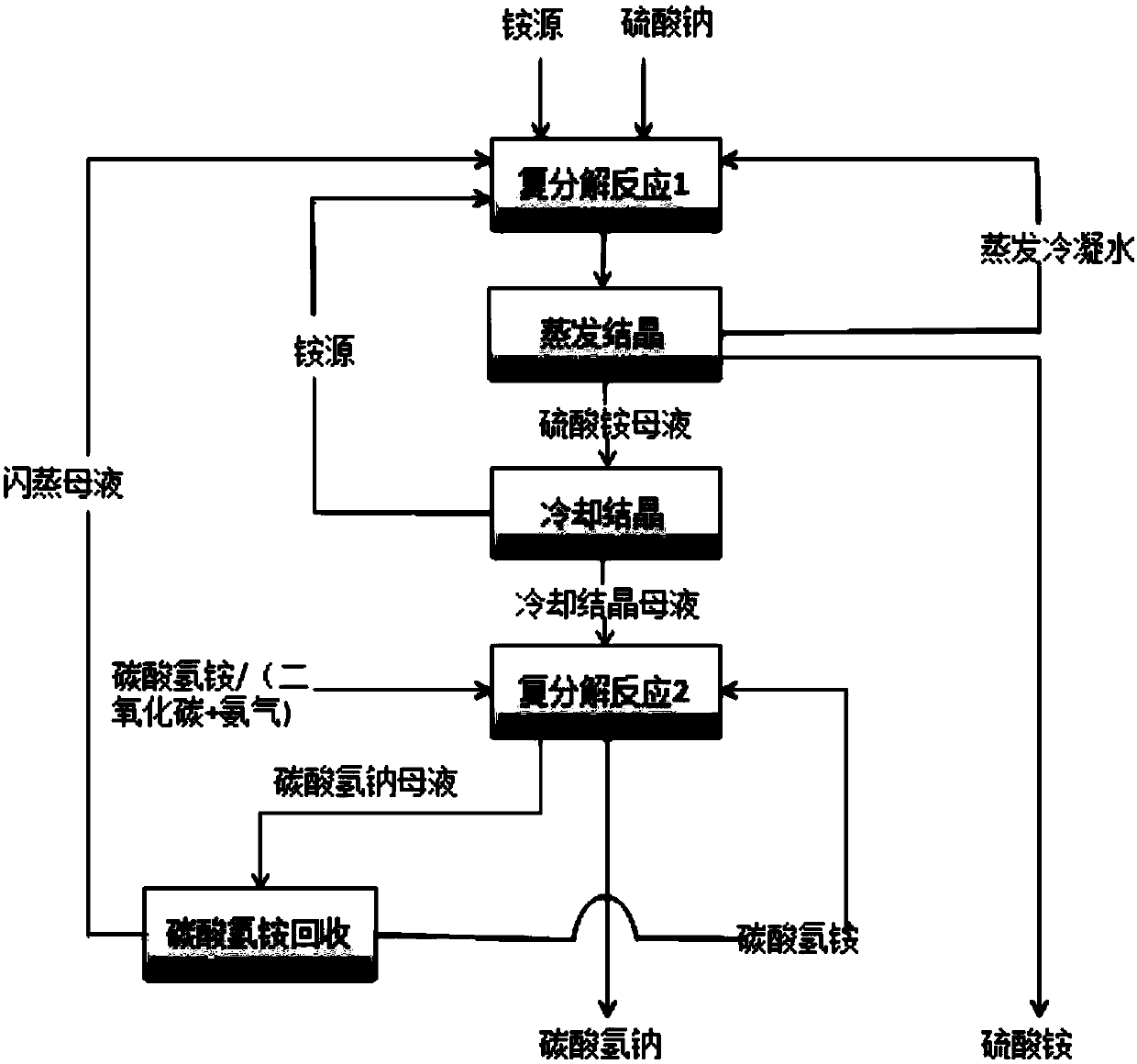
[0060] A kind of method that sodium sulfate prepares ammonium sulfate and sodium bicarbonate, comprises the steps:
[0061] (1) Sodium sulfate is dissolved in optional following evaporation condensed water and the flash evaporation mother liquor that step (4) returns to obtain sodium sulfate solution, and sodium sulfate solution reacts at normal temperature with the ammonium nitrate supplemented and the ammonium nitrate returned by cooling crystallization After the reaction, the solution is subjected to evaporation crystallization and liquid-solid separation to obtain ammonium sulfate crystals and ammonium sulfate mother liquor;
[0062] (2) The ammonium sulfate mother liquor obtained in step (1) is crystallized by cooling to 0° C., and excessive ammonium nitrate is separated out, and ammonium nitrate crystals and crystallization by cooling mother liquor are respectively obtained after liquid-solid separation;
[0063] (3) The cold crystallization mother liquor obtained in ste...
Embodiment 2
[0067] A kind of method that sodium sulfate prepares ammonium sulfate and sodium bicarbonate, comprises the steps:
[0068] (1) Sodium sulfate is dissolved in optional evaporative condensed water and the flash mother liquor returned by step (4) to obtain sodium sulfate solution, and sodium sulfate solution reacts with ammonium nitrate supplemented and ammonium nitrate returned by cooling crystallization at 60°C After the reaction, the solution is subjected to evaporation crystallization and liquid-solid separation to obtain ammonium sulfate crystals and ammonium sulfate mother liquor;
[0069] (2) The ammonium sulfate mother liquor obtained in step (1) is crystallized by cooling to 20° C., and excessive ammonium nitrate is separated out, and ammonium nitrate crystals and crystallization by cooling mother liquor are respectively obtained after liquid-solid separation;
[0070] (3) The cold crystallization mother liquor obtained in step (2) reacts with carbon dioxide and ammonia...
Embodiment 3
[0074] A kind of method that sodium sulfate prepares ammonium sulfate and sodium bicarbonate, comprises the steps:
[0075] (1) Sodium sulfate is dissolved in optional evaporative condensed water and the flash mother liquor returned by step (4) to obtain sodium sulfate solution, and sodium sulfate solution reacts with ammonium nitrate supplemented and ammonium nitrate returned by cooling crystallization at 90°C After the reaction, the solution is subjected to evaporation crystallization and liquid-solid separation to obtain ammonium sulfate crystals and ammonium sulfate mother liquor;
[0076] (2) The ammonium sulfate mother liquor obtained in step (1) is crystallized by cooling to 30° C., and excessive ammonium nitrate is separated out, and ammonium nitrate crystals and crystallization by cooling mother liquor are respectively obtained after liquid-solid separation;
[0077] (3) The cooled crystallization mother liquor obtained in step (2) reacts with ammonium bicarbonate at ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com