Chitosanase mutant and application thereof
A chitosanase and mutant technology, applied in the field of genetic engineering, can solve the problems of enzyme thermal stability, low enzyme activity, and restriction of large-scale industrial development of enzymatic degradation of chitosan, so as to improve enzyme activity And its thermal stability, chitosan enzyme activity is high, the effect of excellent thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] (1) Acquisition of chitosanase gene
[0029] According to the gene sequence of chitosanase BC345 in the genome DNA of human intestinal bacteria Bacteroides clarus YIT 12056 strain (reviewed by GenBank: AFBM00000000.1), codon optimization and gene synthesis were carried out.
[0030] The gene sequence of chitosanase BC345 is shown in SEQ ID NO: 1; the sequence after optimizing the original sequence was completed by Huada Qinglan Biotechnology (Wuxi) Co., Ltd., and the sequence after codon optimization is shown in SEQ ID NO: 2 Shown, the amino acid sequence of this chitosanase gene code is shown in SEQ ID NO:3:
[0031] SEQ ID NO:1:
[0032] ATGAAAACATGGAAAGCAGAAGCCGCCATTATTGCTGTCGGTATGGTTTTGTTGGGAGTGGTAATAAAATGGGGTATAAACGATTTTATAGATAAGGAGCGTATTGTCAGCGTAAAAGGGCTGGCTGAGATGGAAGTCCCCGCCGATAAGGTAATATGGCCTTTGATGTATAAGGATATCGGGAACGACCCGTCTTTGCTTTATGCCAATATGGAGCAGAAGAACAAAGTTATTGTAAAGTTTCTGGAAAGTAACGGCATCGCTAAAGAAGAAATCAGCATTGCTCCGCCCGAAGTGATAGACATGCAGGCCGAACGTTACGGAAACCGTGATAT...
Embodiment 2
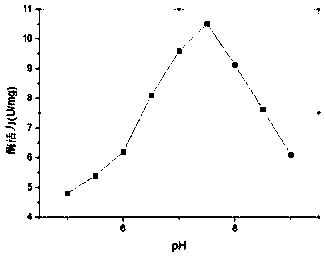
[0088] The determination of embodiment 2 chitosanase BC345 enzymatic properties
[0089] ① The molecular weight of the chitosanase is determined: the purified BC345 protein sample is subjected to discontinuous sodium dodecylsulfonate-polyacrylamide SDS-PAGE gel electrophoresis in a conventional manner, and the results are as follows: figure 2 shown. It is detected as an electrophoresis band by denaturing polyacrylamide gel electrophoresis, and compared with standard protein SDS-PAGE electrophoresis patterns of known molecular weight, the molecular weight of BC345 protein is about 26000 Daltons (26kDa), and the enzyme is a monomeric protein.
[0090] ② Determination of chitosanase activity:
[0091] The activity of chitosanase was determined by dinitrosalicylic acid (DNS) method. Definition of enzyme activity unit: 1U means the amount of enzyme required to release 1 μmol of reducing sugar per minute under the above conditions. In order to measure the Km and Vmax of chitosan...
Embodiment 3
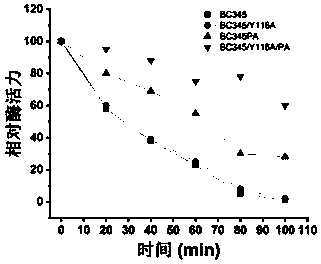
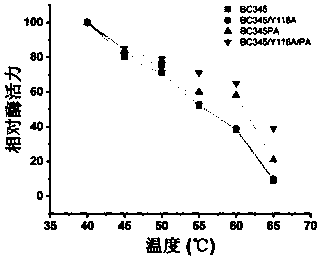
[0100] Construction of chitosanase mutants
[0101] 1) Mutant BC345 / Y116A mutates tyrosine Y at position 116 of the amino acid sequence of chitosanase BC345 to alanine A, as shown in SEQ ID NO: 4 below. The specific implementation method is that the plasmid pPICAɑ-BC345 obtained in the above example is used as a template through the primers of site-directed mutagenesis, and the mutant primers are as follows, and the mutant strain is obtained by PCR.
[0102] The PCR reaction system is as follows:
[0103] Forward primer (10pM) 1μL Reverse primer (10pM) 1μL template DNA 1μL 2×TransStart FastPfu PCR SuperMix 50μL H 2 O
43μL
[0104] PCR amplification conditions were as follows: pre-denaturation, denaturation, annealing, extension, 33 cycles, extension. Length: 27 Upstream primers:
[0105] 5'-AGAGCCAACGCTACTTCTGTTATCACT-3'
[0106] Length: 29
[0107] Downstream primers:
[0108] 5'-AGCGTTGGCTCTGTAAGCGATGTCTCTGT-3'
[0109] ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com