Synergistically modified composite electrocatalyst and application thereof in ethanol oxidation
An electrocatalyst and synergistic modification technology, applied in the field of electrocatalytic materials, can solve the problems of ethanol fuel cell technology development limitations, lack of high-activity anode catalyst, low catalytic activity, etc., and achieve excellent catalytic activity, abundant active sites, and process simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
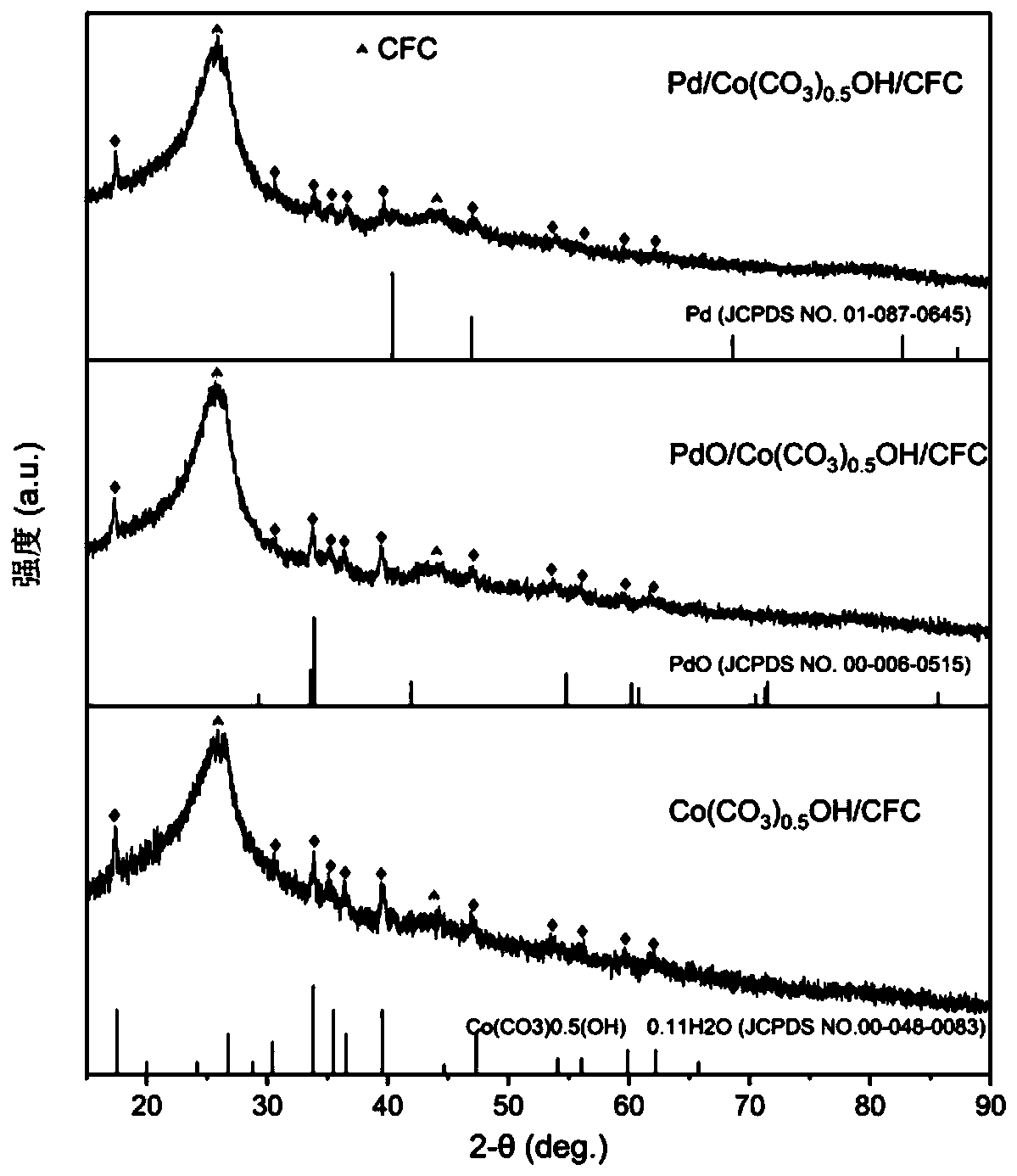
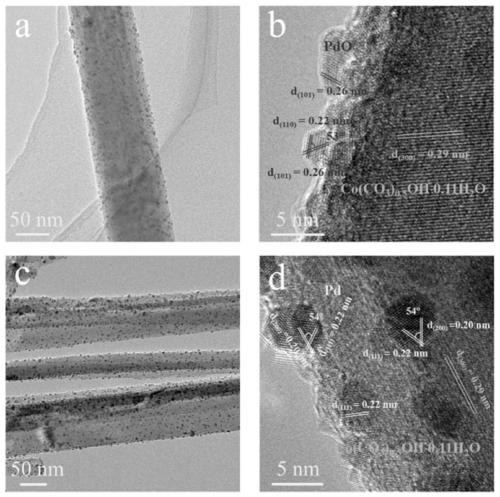
[0042] Pd / Co(CO 3 ) 0.5 Synthesis, characterization and electrocatalytic performance of OH / CFC catalyst:
[0043] (1) Catalyst preparation:
[0044] Using carbon cloth (CFC) as the carrier, its thickness is 0.33mm, and the areal density is ~120g / m 2 . Carbon cloth (1×4cm 2 ) After 10 minutes of ultrasonic cleaning with ethanol, hydrochloric acid solution (1M) and deionized water, together with 40mL containing CoCl 2 ·6H 2 The deionized water solution of O (0.1M), urea (0.5M) and ammonium fluoride (0.2M) was placed in a 50mL hydrothermal kettle, and after constant temperature treatment at 100°C for 10 hours, it was naturally cooled to room temperature to prepare samples After thorough cleaning, vacuum drying was performed at room temperature for 3 hours to obtain a hydrothermal sample; the hydrothermal sample was immersed in 3mL H 2 PdCl 4 (0.001M) in an aqueous solution, kept at a constant temperature of 55°C for 18h, the prepared sample is fully cleaned and vacuum dried at room te...
Embodiment 2
[0054] Pd / (Co,Ni)(CO 3 ) 0.5 Synthesis, characterization and electrocatalytic performance of 0H / CFC catalyst:
[0055] (1) Catalyst preparation: carbon cloth (CC, 1×4cm 2 ) Is the carrier, which is ultrasonically cleaned with hydrochloric acid (1M), absolute ethanol and deionized water for 20 minutes, dried in vacuum at room temperature, and then placed in a hydrothermal kettle containing a transition metal salt solution. The transition metal salt and its concentration used in the hydrothermal reaction process are: CoCl 2 ·6H 2 O(0.04M), NiCl 2 ·6H 2 O (0.02M), urea (0.3M) and ammonium fluoride (0.12M), the hydrothermal reaction condition is 130℃ constant temperature for 15 hours; the hydrothermal sample is immersed in 3mLH 2 PdCl 4 (0.002M) in an aqueous solution, kept at a constant temperature of 65℃ for 16h; 2 It is heated to 160°C in an atmosphere at a heating rate of 10°C / min, and cooled to room temperature after 2 hours of constant temperature treatment to prepare the target ...
Embodiment 3
[0062] Pt / Co(CO 3 ) 0.5 Synthesis and electrocatalytic performance of OH / NF catalyst:
[0063] (1) Catalyst preparation: use foamed nickel (NF, 1×4cm 2 ) Is the carrier, which is ultrasonically cleaned with hydrochloric acid (1M), absolute ethanol and deionized water for 20 minutes, washed and dried with deionized water and absolute ethanol, and then placed in a hydrothermal kettle containing a transition metal salt solution. The transition metal salt and its concentration used in the hydrothermal reaction process are: CoCl 2 ·6H 2 O (0.01M), urea (0.05M), ammonium fluoride (0.03M) hydrothermal reaction conditions are 90 ℃ constant temperature for 5 hours; the hydrothermal sample is immersed in 3mL K 2 PtCl 4 (0.0015M) in the aqueous solution, kept at a constant temperature of 30℃ for 8h; 2 It is heated to 150°C in an atmosphere at a heating rate of 10°C / min, and then cooled to room temperature after 1.5 hours of constant temperature treatment to prepare the target catalyst.
[0064...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com