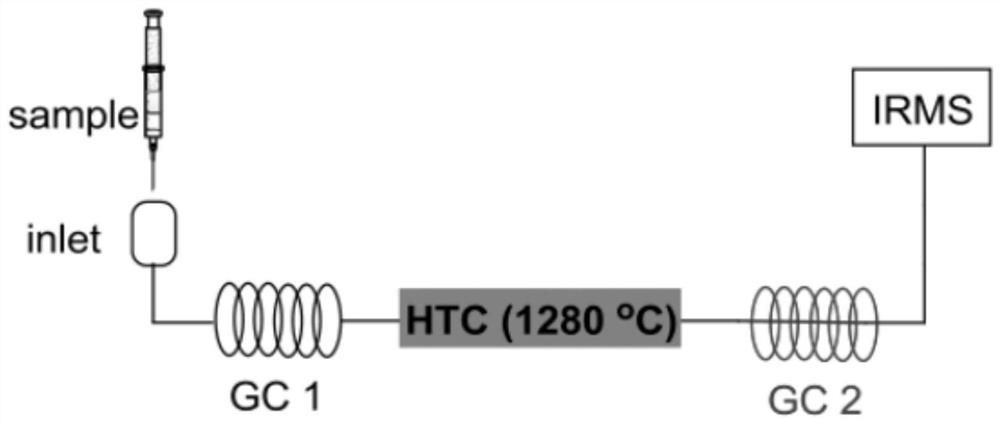
Method for Simultaneous Determination of Nitrogen and Oxygen Isotopic Composition of Natural Nitrate and Nitrite
A technology for nitrite nitrogen and oxygen isotopes, applied in chemical instruments and methods, separation methods, measuring devices, etc., can solve the problems of unsuitable samples, low conversion rate, and lengthy, saving measurement time and reducing sample consumption. , the effect of simplifying the measurement process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
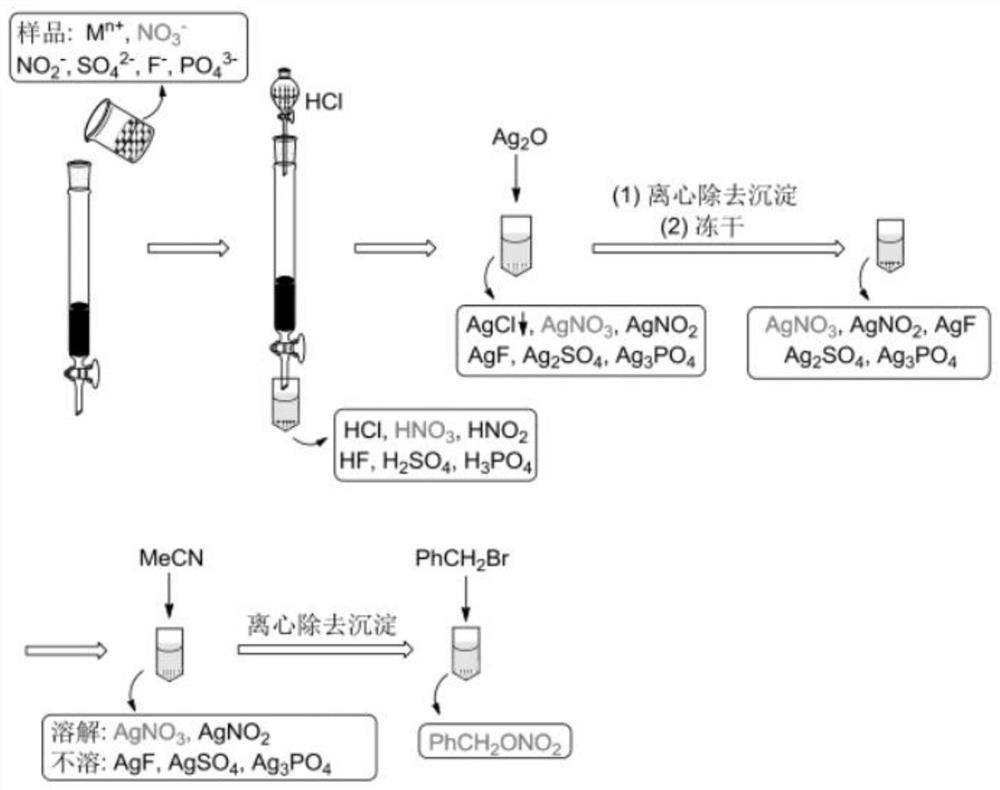
[0079] Take 5ml of Weihe water, filter insoluble impurities with a filter pre-washed with ultrapure water, and then pass through an ion exchange column filled with 0.3ml of anion exchange resin (Bio-Rad: AG1-X8, 200-400 mesh, chlorine type) (column inner diameter 0.6cm) to enrich for nitrate; then use 3M HCl (3.5ml) to elute, and collect the eluent; add Ag to the eluent 2O (about 1.2~1.5g) to pH=6~7 of the reaction solution, filtered to obtain AgNO 3 solution; freeze-dried to obtain AgNO 3 solid; use 0.3 ml of acetonitrile (CH 3 CN) dissolve AgNO 3 Solid (if the sample contains AgNO 2 Also soluble in acetonitrile), the insolubles (usually AgF, Ag 2 SO 4 , Ag 3 PO 4 etc.) are removed by centrifugation.
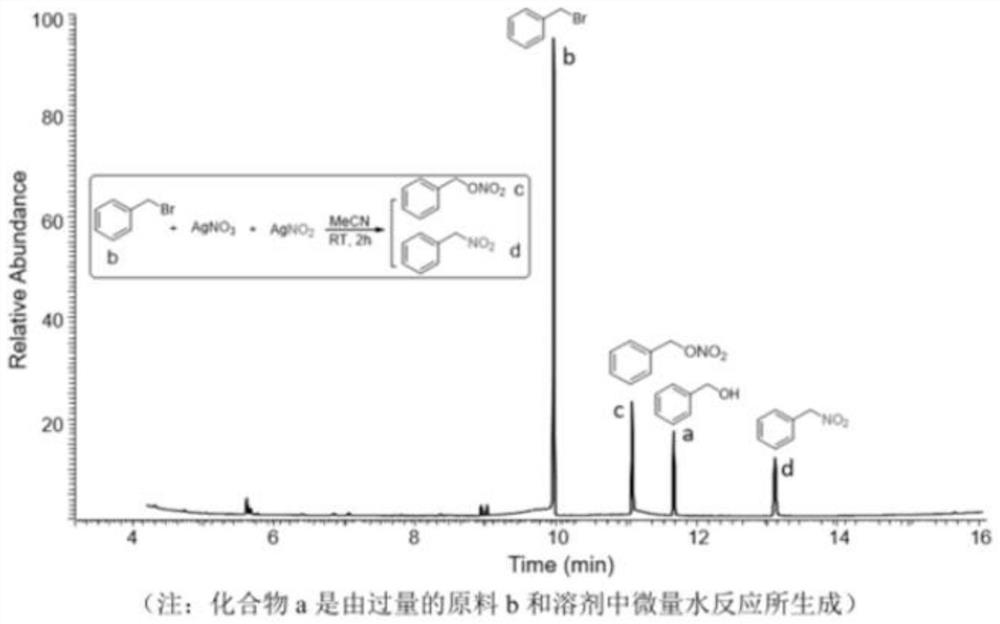
[0080] To the AgNO obtained above 3 Add bromomethylbenzene (CAS: 100-39-0) to the acetonitrile solution, the molar amount of adding bromomethylbenzene should be AgNO 3 and AgNO 2 1.5 times the total molar amount, stirred at room temperature for 2 hours, silver nitrat...
Embodiment 2
[0082] Fresh lettuce was washed with ultrapure water, -18°C to further processing. Weigh 0.5 g of lettuce and grind it in a pre-cleaned mortar to destroy the cell wall, then dissolve it with 6-8 ml of deionized water, and remove the residue by centrifugation or filtration; get the delta of nitrate in leaves 18 O and delta 15 N isotopic composition, the results are as Figure 14 shown.
Embodiment 3
[0084] Take 10g of fresh soil collected in a 250ml conical flask, add 50ml of deionized water, shake on a shaker for 1 hour, sonicate for 30 minutes, and centrifuge after standing for 3 hours to separate the centrifuge, take 10ml of the centrifuge and use the treatment in Example 1. Step processing, and finally get the δ of nitrate in soil 18 O and delta 15 N isotopic composition, the results are as Figure 15 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com