Method for preparing GLP-1 or GLP-1 analogue polypeptides by using escherichia coli to express tandem sequence
A technology of GLP-1 and Escherichia coli, applied in the biological field, can solve the problems of difficult identification of enzyme cleavage sites, low yield of target protein, incomplete enzyme cleavage, etc. Conducive to the effect of enzyme digestion and separation and purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] 1. Sequence design of GLP-1 analogue triple tandem protein 1
[0067] In order to increase the expression level of the GLP-1 analogue precursor peptide in prokaryotic expression and increase the proportion of the precursor peptide chain in the entire expressed peptide chain, according to the above-mentioned patent idea, the GLP-1 analogue precursor peptide chain was subjected to three steps Tandem design (n=3), and GLP-1 analog precursors are connected by trypsin cleavage site or kex2 enzyme cleavage site, in order to make trypsin or kex2 enzyme better recognize trypsin cleavage site Or kex2 enzyme cleavage site, to improve the efficiency of enzyme cleavage, we designed a key auxiliary sequence (m) at the trypsin cleavage site or kex2 enzyme cleavage site. According to the GLP-1 analogs published by NCBI (semaglutide precursor sequence (9-37) and liraglutide precursor sequence (7-37)), at its N-terminal leader peptide MRLNSA, in GLP-1 natural Between the sequence and t...
Embodiment 2
[0083] Example 2: Construction of GLP-1 analog tandem protein recombinant plasmid and construction of engineering bacteria
[0084] The nucleotide sequences corresponding to the amino acid sequences designed above were optimized according to the codons of Escherichia coli, and the codon-optimized gene synthesis was carried out by chemical synthesis, and then ligated into pET32a through BamHI and XhoI to construct four recombinant plasmids . Pass the above recombinant plasmid through CaCl 2 The transformation method was introduced into BL21 (DE3), and the single clones were screened for resistance to construct multiple strains of semaglutide precursor recombinant 3 and 4 tandem protein engineering bacteria. After sequencing, the sequence in the engineering bacteria was consistent with the design.
Embodiment 3
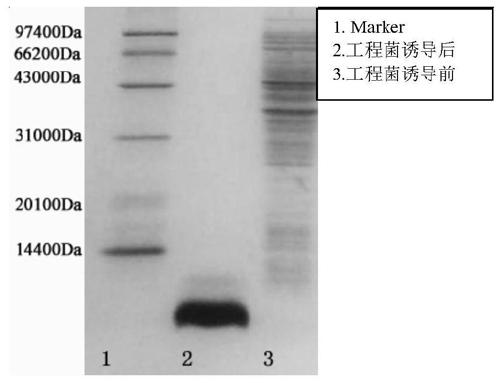
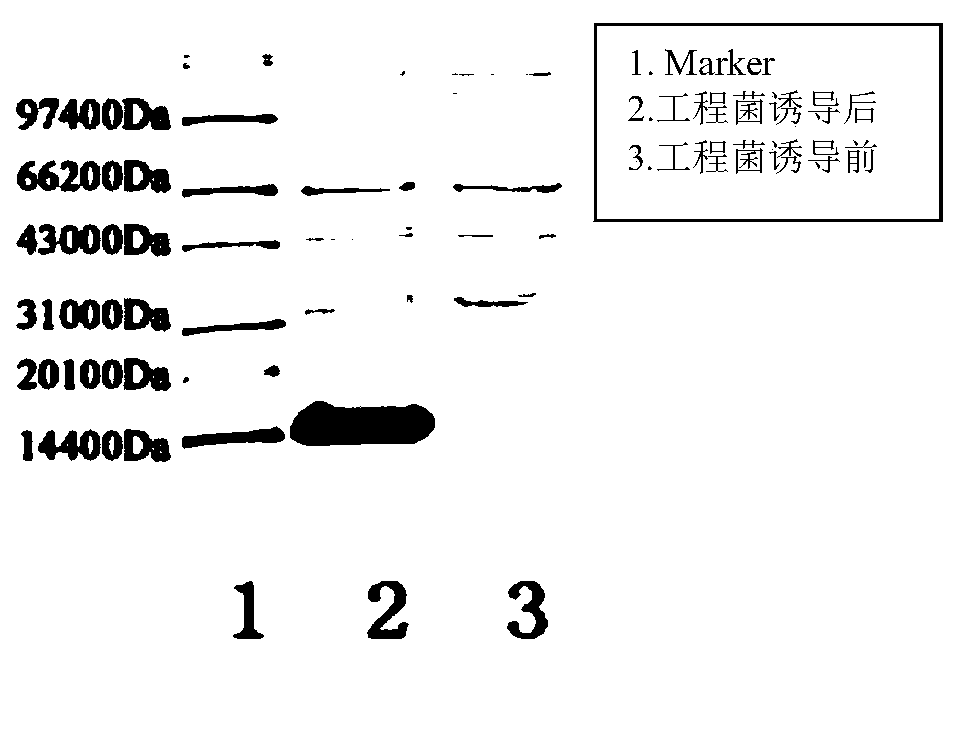
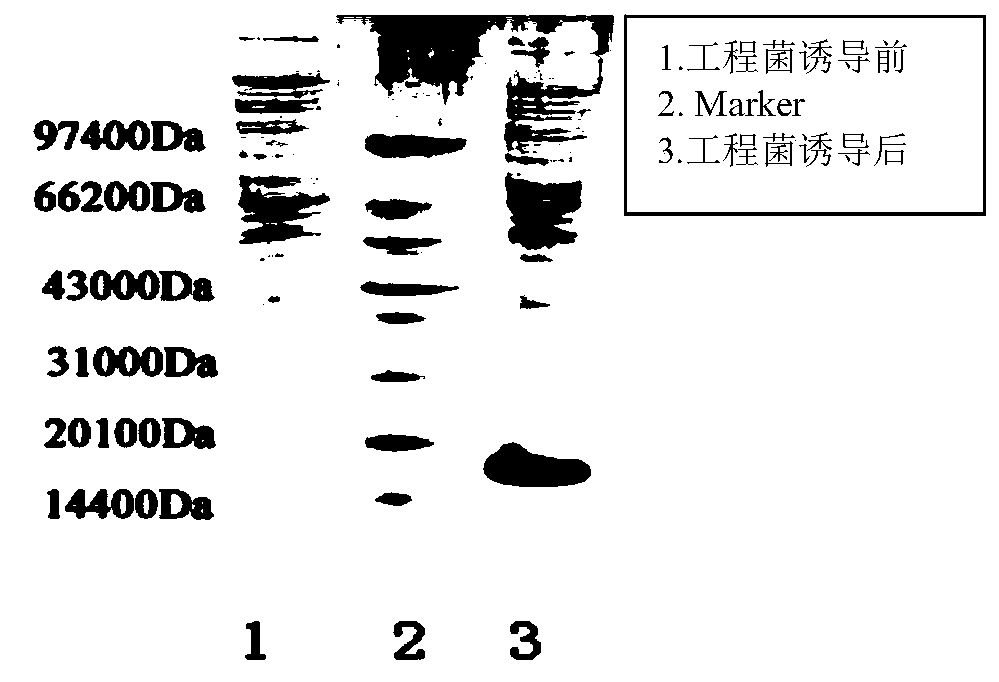
[0085] Example 3: Induced expression of GLP-1 analog tandem proteins
[0086] 3.1 LB medium formula
[0087] Table 3. LB medium formula
[0088]
[0089] Prepare 11L LB culture medium according to Table 3, after dissolving and mixing, absorb 10ml and 1.7L culture medium and put them into 50ml and 3L Erlenmeyer flasks respectively, prepare 6 bottles each, seal and sterilize (121°C, 25min). use.
[0090] 3.2 Recovery of engineered bacteria
[0091] Take out one semaglutide precursor recombinant 3 and 4 tandem protein glycerol bacteria, respectively insert 6 tubes of 10ml sterilized medium according to 1% inoculation amount, and culture overnight on a shaker at 37°C and 250rpm.
[0092] 3.3 Induction culture of engineered bacteria
[0093] Take out the resuscitated bacteria solution, insert 1.7L sterilized medium into 6 mice according to the inoculum amount of 1%, culture on a shaker at 37°C and 250rpm for about 7-8h, add 0.1mM IPTG, and incubate at 37°C Expression was in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com