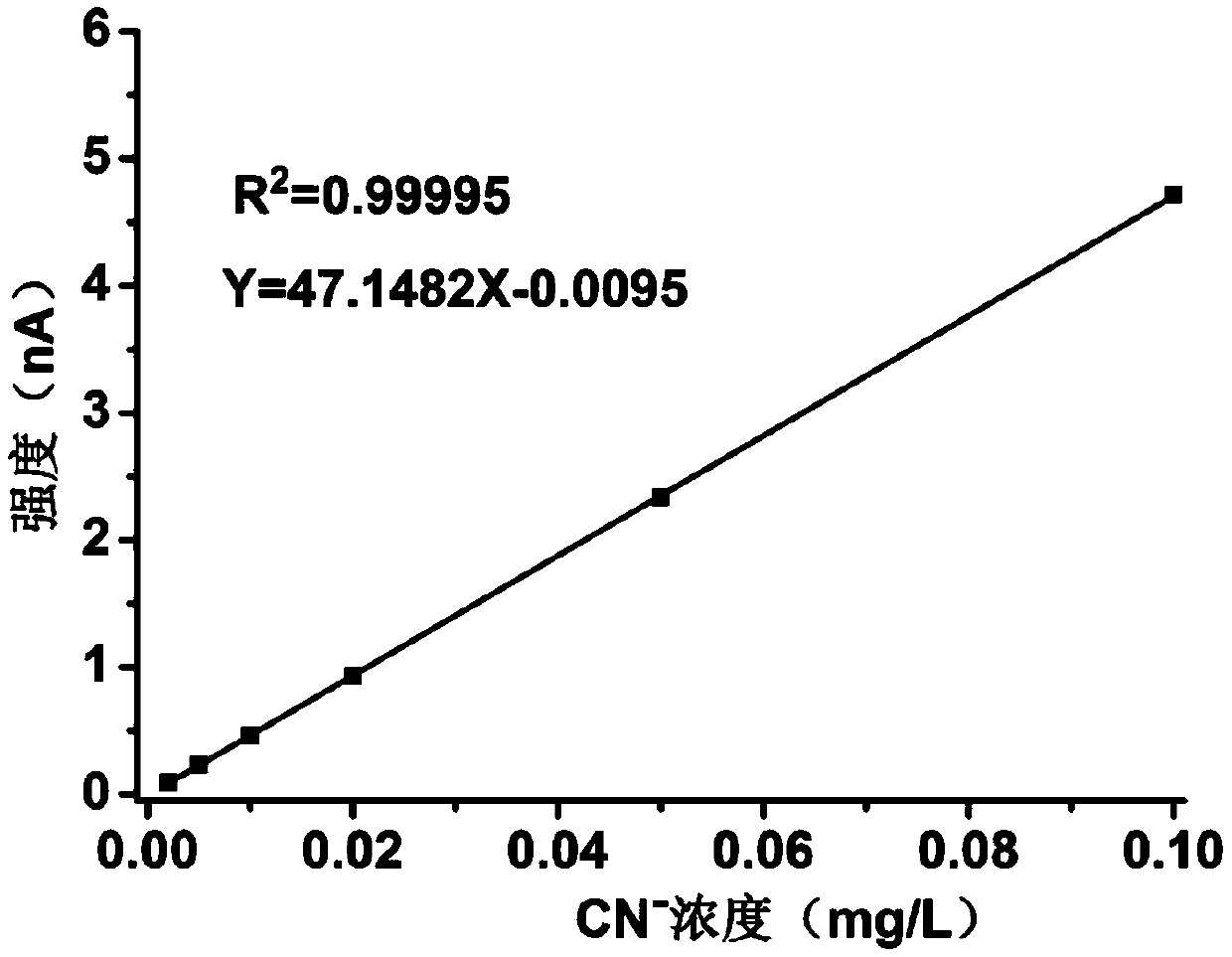
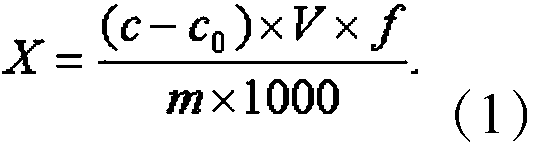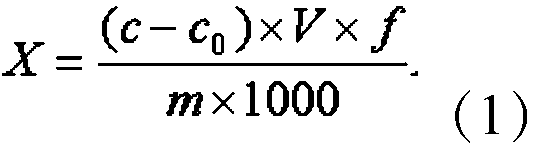Method for determining content of cyanide in electroforming process gold product by ion chromatography
A technology of ion chromatography and gold products, which is applied in the field of detection of new gold products, can solve the problems of poor stability and easy destruction of cyanide, and achieve the effects of high detection amount, improved dissolution amount, and good peak shape
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0060] Select 6 kinds of electroforming process gold product samples, two of which are cyanide-free electroforming process (No. 1#, 2#), and 4 kinds are cyanide electroforming process (3#, 4#, 5#, 6#) . Under the optimal conditions of step 2, step 3, and step 4, weigh 0.2g of the sample and pulverize it, and choose 5 kinds of reagents according to the sample dissolution of step 1, 1-SJ-1#: 50mL aqua regia solution; 1-SJ-2#: 50mL , 50% (volume ratio) aqua regia; 1-SJ-3#: 50mL concentrated hydrochloric acid (analytical pure); 1-SJ-4#: 50mL concentrated sulfuric acid (analytical pure); 1-SJ-5#: 50mL phosphoric acid ( Analytical pure); 1-SJ-6#: the mixed solution of 50mL sulfuric acid and hydrochloric acid (concentration of sulfuric acid in the mixed solution is 10wt%, concentration of hydrochloric acid is 10wt%); 1-SJ-7#: 50mL mixed reagent (40mL water, 10mL (analytically pure nitric acid), 18g sodium chloride, 5g tartaric acid); 1-SJ-8#: 50mL mixed reagent (48mL water, 2mL (ana...
example 2
[0066] Adopt six samples selected in Example 1 to carry out research experiment, take by weighing 0.2g sample pulverization, dissolve sample (1-SJ-8# described condition) according to step 1 optimal condition, step 2 separation reagent selects 5 kinds of reagents, 2-SJ-1#: ethyl acetate; 2-SJ-2#: dichloromethane; 2-SJ-3#: chloroform; 2-SJ-4#: isopropyl ether; 2-SJ-5#: mixed Reagents (30mL ethyl acetate, 35mL dichloromethane, 15mL isopropyl ether); 15mL reagent was extracted and separated, and part of the aqueous phase solution was collected. Under step one, step three, step four optimal conditions, measure the content of cyanide (in cyanide ion), the results are shown in Table 2.
[0067] Table 2. Cyanide content in electroformed gold products under different separation reagent conditions
[0068]
[0069]
[0070] The results show that the cyanide test results of the six selected separation and extraction reagents to separate the cyanide process gold product samples a...
example 3
[0072] The six samples selected in Example 1 were used for research experiments, 0.2g samples were weighed and pulverized, and the samples were dissolved successively according to the preferred conditions of step 1 and step 2, and the cyanide was separated to obtain an aqueous cyanide solution. Transfer the separated aqueous cyanide solution to a round bottom flask, add 6 different extraction reagents, 3-SJ-1#: 15mL hydrochloric acid analytically pure; 3-SJ-2#: 15mL phosphoric acid analytically pure; 3-SJ-3 #: 15g oxalic acid; 3-SJ-4#: 15g tartaric acid; 3-SJ-5#: 15g phthalic acid; 3-SJ-6#: 7g oxalic acid, 8g tartaric acid; 3-SJ-7#: 5g oxalic acid , tartaric acid 8g, o-dibenzoic acid 2g, reflux at 100°C, absorb the distilled fraction with 40mL 30g / L sodium hydroxide aqueous solution to obtain cyanide absorption solution, and dilute to 50mL with 20g / L sodium hydroxide solution ; Under the optimal condition of step 4, measure the content of cyanide (in cyanide ion), the results ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com