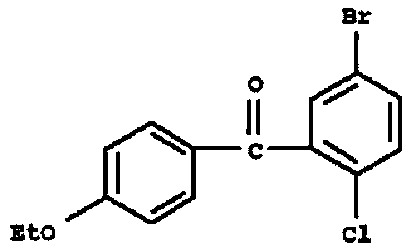
Preparation method of 5-bromo-2-chloro-4'-ethoxybenzophenone
A technology of ethoxybenzophenone and chlorobenzoic acid, which is applied in the field of pharmaceutical synthesis, can solve the problems of affecting product yield, difficult to directly recover and apply acidic waste solvents, and increasing production costs, so as to avoid the generation of acidic solvents, Good reaction effect, the effect of ensuring the conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
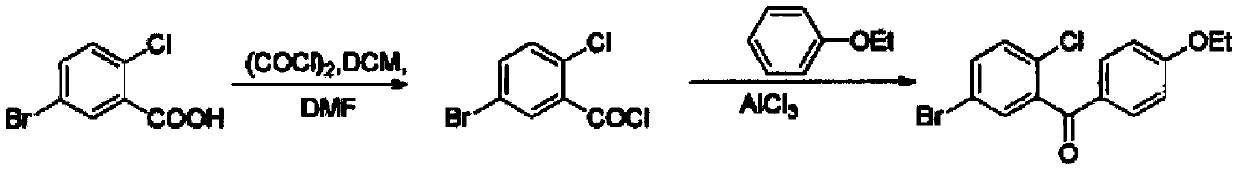
[0026] The preparation method of 5-bromo-2-chloro-4'-ethoxybenzophenone, add thionyl chloride 24g (0.2mol), DMF0.1ml, 5-bromo-2-chlorobenzoic acid successively in the flask 23.5g (0.1mol), stirred and heated to reflux for 4h, after the reaction was completed, the net thionyl chloride was distilled off under reduced pressure at 60°C to obtain 25.1g of crude 5-bromo-2-chlorobenzoyl chloride as a yellow solid (yield 99% );
[0027] Directly add 100ml of dichloromethane and stir to dissolve, then lower the temperature to -20℃~-25℃, add 66g of aluminum trichloride supported on silica gel (the loading capacity is 1.6mmol / g, a total of 0.105mol), and control the vacuum degree of the system to -0.05MPa 13.4g (0.11mol) of phenetole was added dropwise to react for 2 hours, filtered, soaked and washed with 40ml of dichloromethane, and merged into the filtrate, then washed with 5% sodium bicarbonate solution and water, and evaporated to remove the solvent The resulting solid is recrystal...
Embodiment 2
[0029] The preparation method of 5-bromo-2-chloro-4'-ethoxybenzophenone, add thionyl chloride 48g (0.4mol), DMF0.1ml, 5-bromo-2-chlorobenzoic acid successively in the flask 23.5g (0.1mol), stirred and heated to reflux for 2h, after the reaction was over, thionyl chloride was distilled off under reduced pressure at 60°C to obtain 24.9g of crude 5-bromo-2-chlorobenzoyl chloride as a yellow solid (yield 98% );
[0030] Directly add 100ml of dichloromethane and stir to dissolve, then lower the temperature to -10°C~-15°C, add 66g of aluminum trichloride supported on silica gel (the loading capacity is 1.6mmol / g, a total of 0.105mol), and control the vacuum degree of the system to -0.03MPa 13.4g (0.11mol) of phenetole was added dropwise to react for 3 hours, filtered, soaked and washed with 40ml of dichloromethane and merged into the filtrate, then washed with 5% sodium bicarbonate solution and water successively, evaporated Solvent; the resulting solid is recrystallized with a mix...
Embodiment 3
[0032] The preparation method of 5-bromo-2-chloro-4'-ethoxybenzophenone, add thionyl chloride 24g (0.2mol), DMF0.1ml, 5-bromo-2-chlorobenzoic acid successively in the flask 23.5g (0.1mol), stirred and heated to reflux for h, and after the reaction was completed, the thionyl chloride was distilled off under reduced pressure at 60°C to obtain 25.2g of the crude product of yellow solid 5-bromo-2-chlorobenzoyl chloride (yield 99% );
[0033] Directly add 100ml of dichloromethane and stir to dissolve, then lower the temperature to -25°C to -30°C, add 66g of aluminum trichloride supported on silica gel (the loading capacity is 1.6mmol / g, a total of 0.105mol), and control the vacuum degree of the system to -0.07MPa 13.4g (0.11mol) of phenetole was slowly added dropwise to react for 4 hours, filtered, soaked and washed with 40ml of dichloromethane, and merged into the filtrate, then washed with 5% sodium bicarbonate solution and water, and evaporated to dryness The solvent obtained 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com