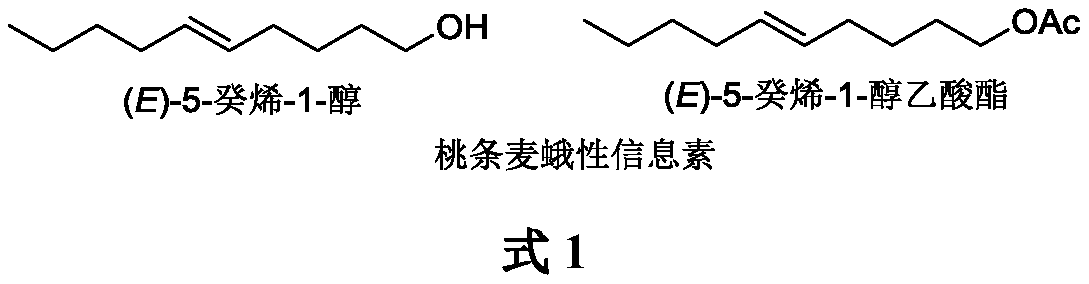
Method for synthesizing sex pheromone of anarsia lineatella
A synthetic method, the technology of P. chinensis, applied in chemical instruments and methods, the preparation of organic compounds, the preparation of hydroxyl compounds, etc., can solve the problems of harsh reaction conditions, lengthy reaction routes, and high toxicity of reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
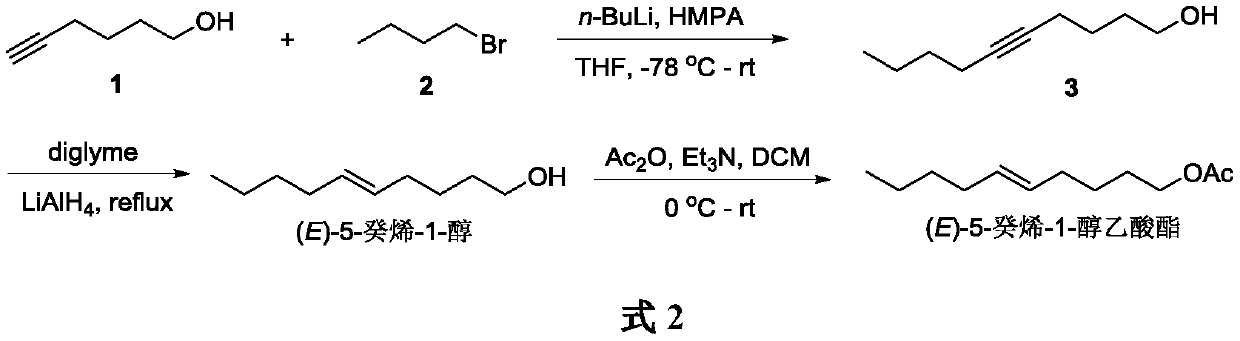
Embodiment 1
[0020] Synthesis of 5-Decyn-1-ol 2
[0021] Under argon protection, 5-hexyn-1-ol 1 (0.20 g, 2 mmol), HMPA (1 mL, 6 mmol) and tetrahydrofuran (10 mL) were sequentially added into a 50 mL Schlenk tube, and stirred evenly. The temperature of the mixture was lowered to -78°C, and n-butyllithium (1.7mL, 2.4M, 4mmol) was slowly added dropwise. After dropping, the mixture was slowly warmed to -30°C and stirred for 1 h. Then 1-bromobutane 2 (0.27 g, 2 mmol) was slowly added dropwise. After the drop, the reaction solution was slowly warmed up to room temperature, and the stirring reaction was continued for 10 h. After the reaction was completed, saturated ammonium chloride aqueous solution (10 mL) was added dropwise under ice cooling to quench the reaction. The layers were separated, the aqueous phase was extracted with ether (3×10 mL), and the organic phases were combined. The organic phase was washed with water (20 mL) and saturated aqueous sodium chloride solution (20 mL), dried ...
Embodiment 2
[0023] Synthesis of (E)-5-decen-1-ol
[0024] Under argon protection, add lithium aluminum hydride (1.48g, 38.88mmol), diethylene glycol dimethyl ether (40mL) and acetylenic alcohol 3 (0.93g, 6.48mmol) into a 100mL three-necked reaction flask equipped with a reflux condenser , the reaction mixture was heated to reflux, and the stirring reaction was continued for 14h. After the reaction, the reaction mixture was cooled to room temperature. Under cooling in an ice bath, aqueous sodium hydroxide solution (5 mL) was added dropwise to quench the reaction. The reaction mixture was suction filtered through celite, and the filter cake was washed with diethyl ether (500 mL). The filtrate was washed with water (3×100mL) and saturated aqueous sodium chloride solution (2×100mL), dried over anhydrous sodium sulfate and concentrated under reduced pressure to obtain light yellow liquid compound (E)-5-decen-1-ol (0.94 g, yield 93%). 1 H NMR (300MHz, CDCl 3 )δ5.42–5.38(m,2H),3.64(t,J=6.5H...
Embodiment 3
[0026] Synthesis of (E)-5-Decen-1-ol Acetate
[0027] Under argon protection, (E)-5-decen-1-ol (0.90 g, 5.75 mmol), dichloromethane (15 mL) and triethylamine (3.49 g, 34.5 mmol) were added to a 50 mL Schlenk tube, and the Acetic anhydride (1.76 g, 17.25 mmol) was slowly added dropwise with bath cooling. The reaction solution was warmed up to room temperature, and the stirring reaction was continued for 8h. After the reaction was completed, water (10 mL) was added to quench the reaction. The layers were separated, the aqueous phase was extracted with dichloromethane (3×15 mL), and the organic phases were combined. The organic phase was washed with water (3×20 mL) and saturated aqueous sodium chloride (30 mL), dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain a crude product. The crude product was purified by silica gel column chromatography (petroleum ether / ethyl acetate 80:1) to obtain light yellow liquid compound (E)-5-decen-1-ol acetat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com