Preparation method and application of recombinant EBV gHgL immunogen
An immunogen and protein technology, applied in the field of biomedicine, can solve the problem of low yield and achieve the effect of large expression, good purity and uniformity, and good natural fidelity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The construction of embodiment 1 recombinant gH and gL expression vector
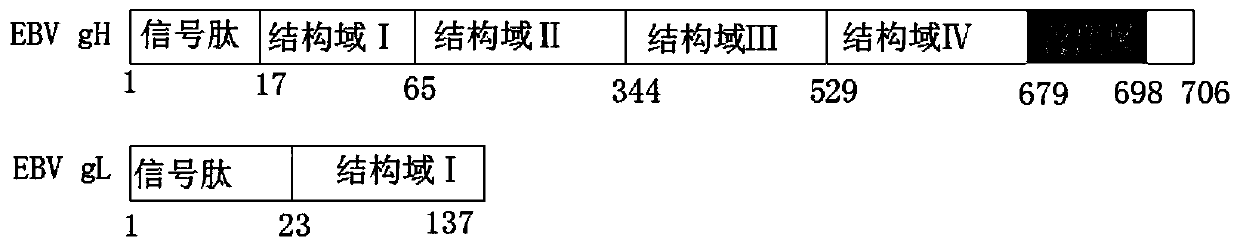
[0028] Using the gH and gL cDNAs synthesized by Jinweizhi Biotechnology Co., Ltd. with codon-optimized codons according to the codon preference of Drosophila as templates, the extracellular domain of gH and the full length of gL were obtained by PCR technology (see figure 1 ) DNA fragment.
[0029] The primers used in the vector construction process are:
[0030] pMT EBV gH Bgl II Forward: GAAGATCTGCCTCCCTCTCCGAGGTCAAAC, SEQ ID NO. 1;
[0031] pMT EBV gH Xba I Reverse: GCTCTAGAGTGGGCACGTTCTTCGTAGAG, SEQ ID NO. 2;
[0032] pMT EBV gL Bgl II Forward: GAAGATCTAACTGGGCCTATCCGTGCTGTCAC, SEQ ID NO. 3;
[0033] pMT EBV gL Xba I Reverse: GCTCTAGAGCCACCTCGATGCCAAGCGTAC, SEQ ID NO.4.
[0034] The PCR reaction system is as follows:
[0035]
[0036] The reaction procedure is as follows:
[0037]
[0038] The two target gene fragments were respectively connected to the modified pMT / Bip / TEV-HisA v...
Embodiment 2
[0045] Example 2 Three-plasmid co-transfection of Drosophila melanogaster S2 cells and screening of S2 stable cell lines expressing target protein
[0046] S2 cells were plated in T25 culture flask one day in advance, and co-transfection was carried out by liposome method when the confluency of the cells reached 60-70%. The transfection step was carried out according to the instructions of the transfection reagent Cellfectin II, the mass ratio of the two recombinant plasmids and the resistance selection plasmid pCoBlast was 19:19:1, and the ratio of gH and gL could be changed to obtain optimal expression, but the two Together, the ratio to the screening plasmid was 38:1. Place the transfected cells in a 27°C biochemical incubator and incubate for 5 hours, discard the transfection solution, add 5 mL of fresh SFX-Insect medium, continue to cultivate for 48 hours, discard the original medium, and add The SFX-Insect medium with blasticidin (final concentration: 25 μg / mL) was used...
Embodiment 3
[0047] Expression and purification of embodiment 3 recombinant gHgL
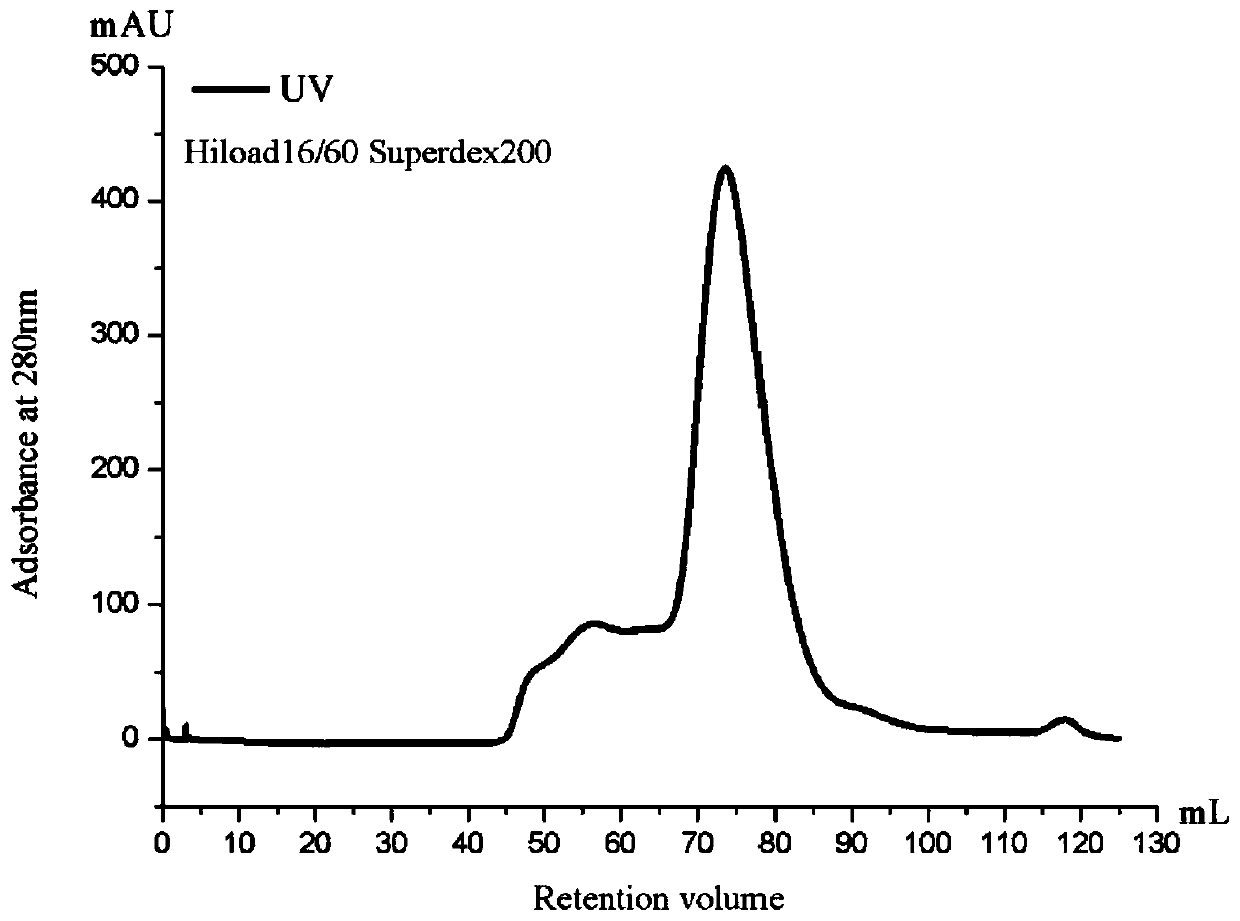
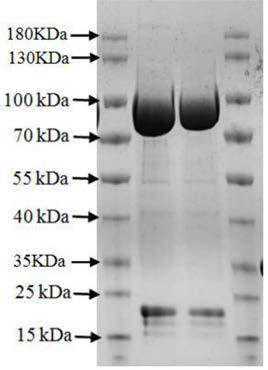
[0048] The selected T25 cultured S2 stable cell line was expanded into a T75 culture flask with a volume ratio of 1:5, and further expanded into a 500mL spinner bottle with a volume ratio of 1:5 for culture, at 27°C, 120- 130rpm, culture for 2-3 days, when the cell density reaches 4-6×10 6 , adding copper sulfate at a final concentration of 0.5 mM to induce the expression of the recombinant protein, and collecting the cell supernatant after 3 days. Pour the S2 cells into a 500mL centrifuge bucket, centrifuge at 4000rpm, 4°C for 15min. The cell supernatant was collected, filtered through a 0.22 μm microporous membrane, transferred to an Amicon Stirred Cell 8003 ultrafiltration cup for concentration, compressed to about 50 mL, and combined with a nickel column buffer (50 mM Tris-HCl, 500 mM NaCl, pH8.0) was diluted 10 times, continued to compress to 40mL, collected the protein solution into a high-speed cent...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com