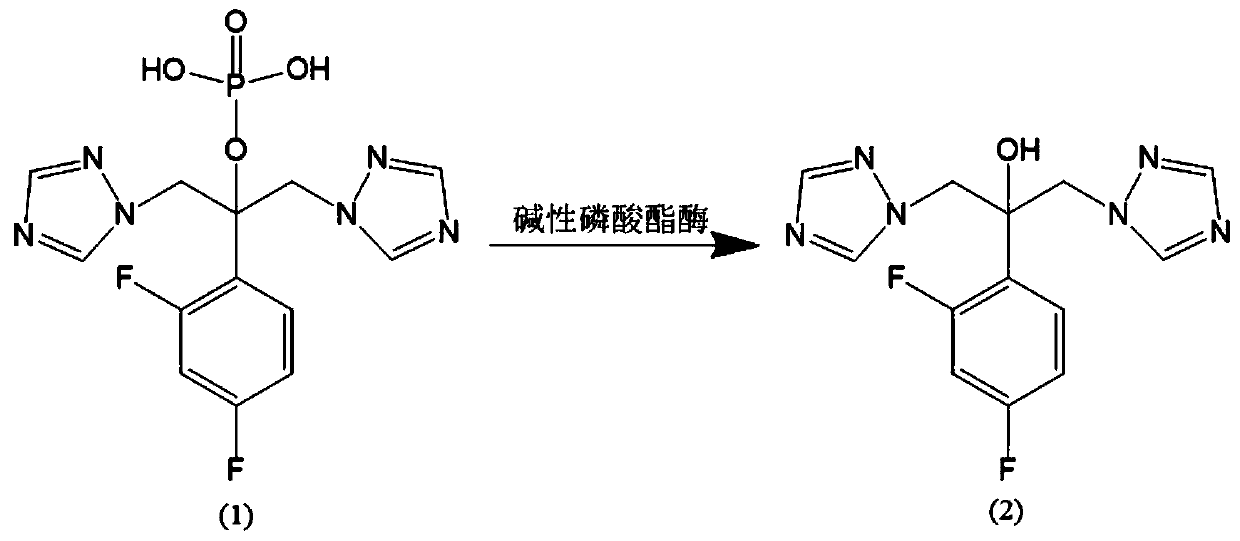
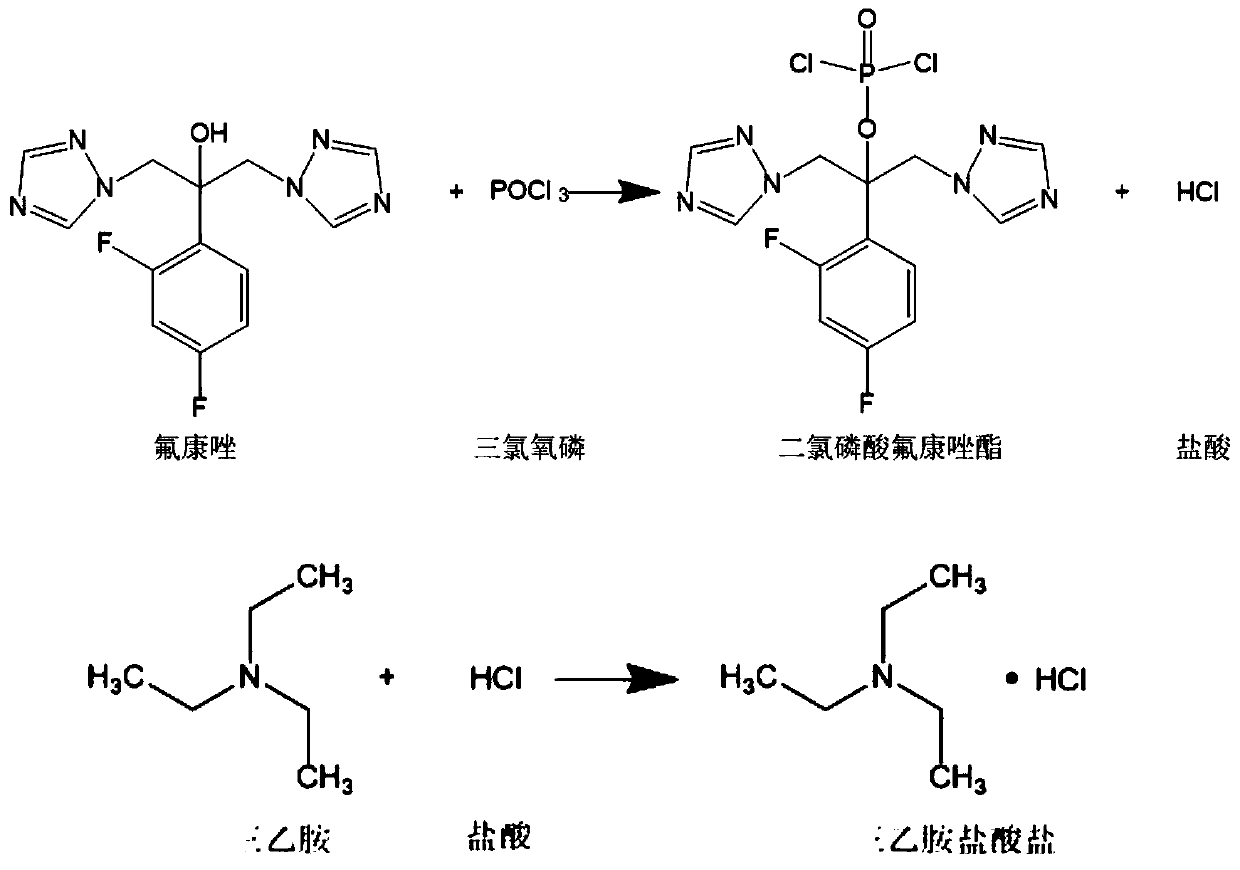
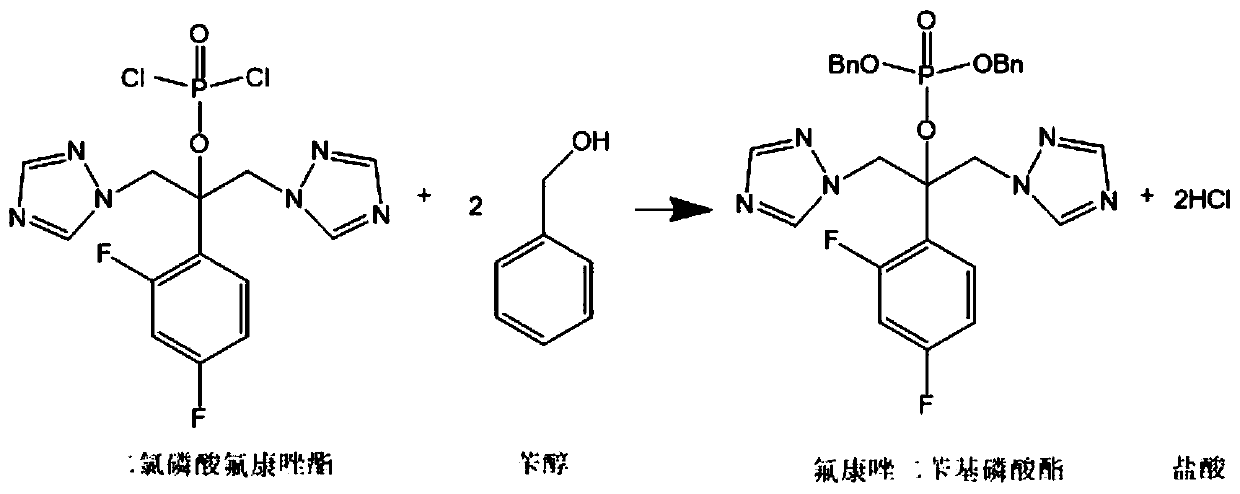
Preparation method of fosfluconazole
A technology of fosfluconazole and fluconazole, which is applied in the field of drug synthesis, can solve the problems of excessive organic waste, environmental pollution, and low yield, and achieve the effects of reducing organic impurities, improving purity, and increasing yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0069] Group 1-1
[0070] Under the protection of nitrogen, control the temperature at -10°C, slowly add 76.7g of phosphorus oxychloride dropwise to 300ml of 3.33mol / L triethylamine in dichloromethane solution, after the drop, maintain the temperature at -10°C and stir for 1 hour, slowly Add 1L of 0.5mol / L fluconazole dichloromethane solution dropwise, and monitor by TLC (mobile phase ethyl acetate:petroleum ether=2:1 mixed solution) the reaction is complete; maintain the temperature at -10°C, add 650ml of water and stir for 30 minutes, Sodium hydroxide was added until the pH of the aqueous phase was 10, and the layers were allowed to stand to separate. The upper aqueous phase was separated, and the organic phase was evaporated to remove the solvent under reduced pressure to obtain solid fluconazole dichlorophosphate.
[0071] Group 1-2
[0072] Under the protection of nitrogen, control the temperature at -5°C, slowly add 161.0 g of phosphorus oxychloride dropwise to 600ml of...
experiment example 2
[0087] Group 3-1
[0088] Under nitrogen protection, control the temperature at 0°C, and slowly add 50.6g of phosphorus oxychloride dropwise to 200ml of 3.3mol / L triethylamine in dichloromethane solution. 0.5mol / L fluconazole dichloromethane solution 600ml, TLC monitoring (mobile phase ethyl acetate:petroleum ether=2:1 mixed solution) the reaction is complete; maintain the temperature at 0°C, add 400ml of water and stir for 30 minutes, add hydroxide Potassium until the pH of the aqueous phase is 8, let stand to separate layers, separate the upper aqueous phase, evaporate the organic phase to remove the solvent under reduced pressure, and obtain solid fluconazole dichlorophosphate.
[0089] Group 3-2
[0090] Under nitrogen protection, control the temperature at 0°C, and slowly add 50.6g of phosphorus oxychloride dropwise to 200ml of 3.3mol / L triethylamine in dichloromethane solution. 0.5mol / L fluconazole dichloromethane solution 600ml, TLC monitoring (mobile phase ethyl acet...
experiment example 3
[0093] Group 4-1
[0094] Under the protection of nitrogen, the temperature is controlled at 0°C, and 50.6g of phosphorus oxychloride is slowly added dropwise to 200ml of 3.3mol / L pyrimidine in dichloromethane. / L fluconazole dichloromethane solution 600ml, TLC monitoring (mobile phase ethyl acetate:petroleum ether=2:1 mixed solution) the reaction is complete; maintain the temperature at 0°C, add 400ml water and stir for 30 minutes, add sodium hydroxide to The pH of the aqueous phase was 8, and the layers were separated after standing. The upper aqueous phase was separated, and the organic phase was evaporated to remove the solvent under reduced pressure to obtain solid fluconazole dichlorophosphate.
[0095] Group 4-2
[0096] Under nitrogen protection, control the temperature at 0°C, slowly add 50.6 g of phosphorus oxychloride dropwise to 200 ml of a 3.3 mol / L N,N-diisopropylethylamine (DIEA) solution in dichloromethane, and maintain the temperature after the drop is comple...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com