Human recombinant interferon fusion gene obtained based on gene modification, fusion protein and preparation method thereof
A technology of fusion protein and fusion gene, which is applied to the preparation method of peptides, methods based on microorganisms, cytokines/lymphokines/interferons, etc., and can solve the problems of no biological activity, affecting production efficiency and production cost, and renaturation. Problems such as difficulty in purification and purification, to achieve the effect of simplified purification steps and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042]A histidine tag and glutamic acid-lysine repeating unit were introduced into the gene sequence of the original human recombinant interferon IFN-β1b (GenScript Biotech Corporation) by PCR amplification, and a human recombinant interferon IFN-β based on genetic modification was obtained. A fusion protein, the nucleotide sequence of the fusion protein is shown in SEQ ID NO.1, and the amino acid sequence is shown in SEQ ID NO.2.
[0043] The PCR amplification reaction system is as follows: first, 2.5 μL of the gene sequence SEQ ID NO.3 with 50 repeated KE amino acid units is used as template DNA, 0.5 μL of the gene sequence of SEQ ID NO.4: CATATGGAAAACCTGTATTT as the upstream primer, and 0.5 μL of the gene sequence of SEQ ID NO. .5: CTTTTTCTTAAG CTACTA CTATTT was used as a downstream primer and made up to 50 μL with distilled water. The reaction process is as follows: pre-denaturation at 94°C for 5 min, denaturation at 94°C for 1 min, annealing at 5°C for 30 s, extension a...
Embodiment 2
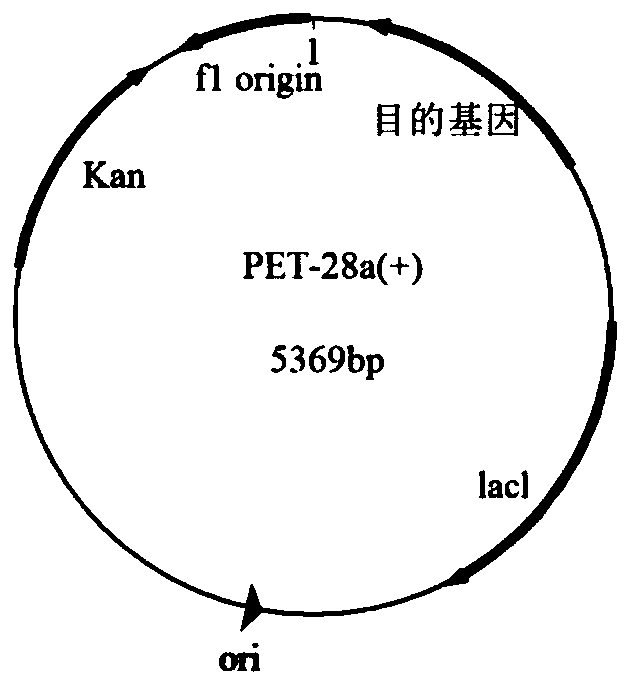
[0045] The IFN-KE50 fusion gene obtained in Example 1 and 2 μg of the PET-28a(+) plasmid (Solarbio | product number: P3110) were digested with restriction endonucleases NdeI and NotI, respectively. The reaction system is: 1.5 μL Nde Ⅰ enzyme, 1.5 μL Not Ⅰ enzyme, 5 μL 10×Buffer, 2-5 μg pET-28a(+) plasmid or IFN-KE50 fusion gene, ddH 2 Make up to 50 μL with O, and bathe in water at 37°C for 30 minutes. The digested IFN-KE50 fusion gene and pET-28a(+) plasmid were recovered from the agarose gel, ligated with T4 ligase at 16°C for 4 hrs to obtain the IFN-KE50 fusion plasmid and placed at room temperature for 2 hrs, and the IFN-KE50 fusion plasmid was transfected To Escherichia coli competent cells (BL21(DE3)), spread on LB plates containing 50 μg / mL kanamycin, and culture overnight at 37°C upside down. Pick colonies on the plate and place them in 50 mL of LB liquid medium containing kanamycin, the concentration of kanamycin is 50 μg / mL, culture at 37°C for 12 hours and expand cu...
Embodiment 3
[0047] According to the volume ratio of 1 to 100, take 3 mL of Escherichia coli liquid transfected with the IFN-KE50 fusion gene in 300 mL of 2×YT medium, add kanamycin to make the concentration 50 μg / mL, and store at 37 ° C. 1. After culturing for 2.5 hours, add IPTG inducer to make the concentration in the culture medium 0.1mM / L, and culture and express at 16°C for 12 hours to obtain Escherichia coli liquid containing IFN-KE50 fusion protein.
[0048] After adding the IPTG inducer according to the above steps, culture and express at 37°C for 12 hours, and the rest of the steps remain unchanged to obtain the Escherichia coli liquid containing the IFN-KE50 fusion protein induced at 37°C.
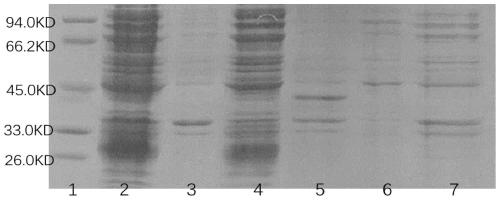
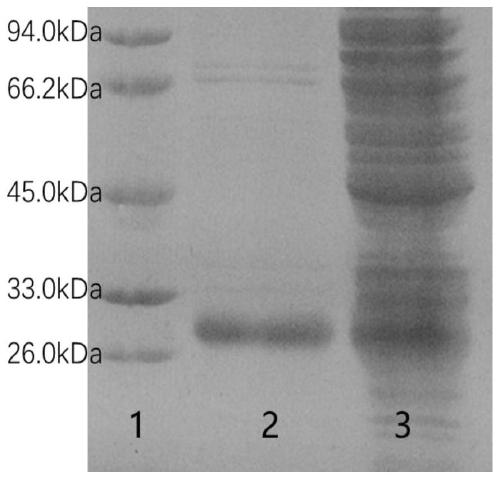
[0049] The SDS-PAGE electrophoresis of described IFN-KE50 fusion protein is as follows figure 1 As shown, the expression under two different temperature conditions was investigated, among which 1 is Marker; 2 is 16 ℃ IFN-KE50 supernatant; 3 is 16 ℃ IFN-KE50 precipitation; 4 is 37 ℃ IFN-KE50 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com