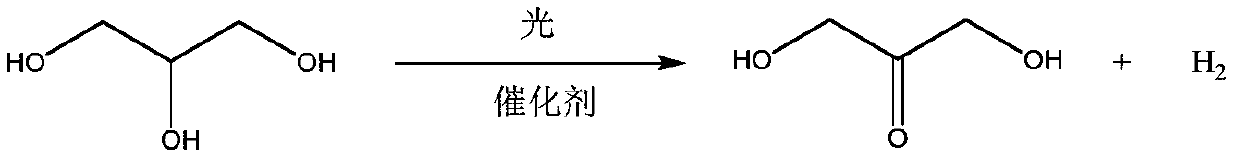
Bifunctional catalyst for photocatalytic synthesis of dihydroxyacetone and hydrogen, preparation method and application thereof
The technology of a bifunctional catalyst and dihydroxyacetone is applied in the field of photocatalytic synthesis of bifunctional catalysts for dihydroxyacetone and hydrogen and the preparation thereof, and can solve the problems of easy polymerization of photogenerated electron-holes, poor photocatalytic performance, and the like, To achieve the effect of good selectivity, simple preparation method and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] 2 g of ammonium acetate and 20 g of urea were added to the mortar. After grinding for 20 minutes, transfer the obtained white powder to a 50mL crucible, compact it, cover the crucible lid and wrap it tightly with tin foil. Put the crucible above into a muffle furnace for firing, the heating rate is 8°C per minute, the firing temperature is 550°C, and the firing time is 2 hours. After the calcination, it was naturally cooled to room temperature, and the yellow solid obtained in the crucible was taken out and ground to powder. The yellow powder obtained above was subjected to secondary roasting: the yellow powder was transferred to a 50 mL crucible, and put into a muffle furnace without the lid of the crucible. The heating rate was 5°C per minute, the firing temperature was 520°C, and the firing time was 2 hours. After the muffle furnace was cooled to room temperature, the yellow powder in the crucible was taken out to obtain a bifunctional catalyst.
Embodiment 2
[0023] Add 1 g of ammonium oxalate and 20 g of urea in a mortar. After grinding for 15 minutes, transfer the resulting white powder to a 50mL crucible, compact it, cover the crucible lid and wrap it tightly with tin foil. Put the crucible above into a muffle furnace for firing, the heating rate is 5°C per minute, the firing temperature is 500°C, and the firing time is 4 hours. After the calcination, it was naturally cooled to room temperature, and the yellow solid obtained in the crucible was taken out and ground to powder. The yellow powder obtained above was subjected to secondary roasting: the yellow powder was transferred to a 50 mL crucible, and put into a muffle furnace without the lid of the crucible. The heating rate was 2°C per minute, the firing temperature was 500°C, and the firing time was 4 hours. After the muffle furnace was cooled to room temperature, the yellow powder in the crucible was taken out to obtain a bifunctional catalyst.
Embodiment 3
[0025] The difference from Example 1 is that the carbon nitride precursor is replaced by cyanamide.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com