Near-infrared light-controlled bistable-state field effect transistor polymer and preparation method and application thereof
A technology of polymers and compounds, applied in the field of polymer materials, can solve problems affecting the stability of organic polymers, semiconductor device functions, etc., achieve excellent carrier transport performance, excellent solubility performance, and avoid damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
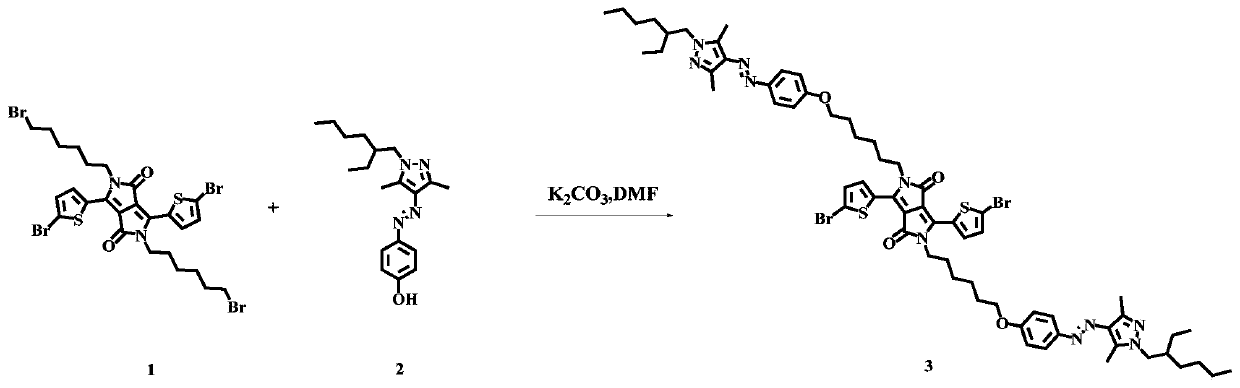
[0063] Synthesis of the compound shown in embodiment 1, formula II (in its formula II, R 1 and R 2 Both are 2-octyldodecyl):
[0064] chemical reaction flow chart figure 1 Shown, concrete reaction step condition is as follows:
[0065] Dissolve compound 1 (1.28mmol) in 50mL DMF, add compound 2 (3.2mmol), react at 60°C for 3h, and stop the reaction. The solvent was removed by a rotary evaporator, and the product 3 (0.136 mmol, yield: 10.6%) was obtained by separation on a silica gel column;
[0066] The structural confirmation data are as follows:
[0067] 1 H NMR (300MHz, CDCl 3 )δ8.695(d, J=3.0Hz, 2H); 8.01-7.97(m, 8H); 7.24(d, J=3.0Hz, 2H); 7.02-6.97(m, 8H); 4.06-3.94(m ,8H); 3.94-3.92(d,J=6.0Hz,4H); 2.55(s,6H); 2.50(s,6H); 2.00-1.96(m,2H); 1.87-1.74(m,10H); 1.55-1.26(m,24H); 0.97-0.89(m,12H).;
[0068] HR-MS: The calculated value is C 66 h 79 Br 2 N 6 o 6 S 2 (M + ): 1273.3863, mass spectrum peak position: 1273.3861.
[0069] From the above, it can be seen ...
Embodiment 2
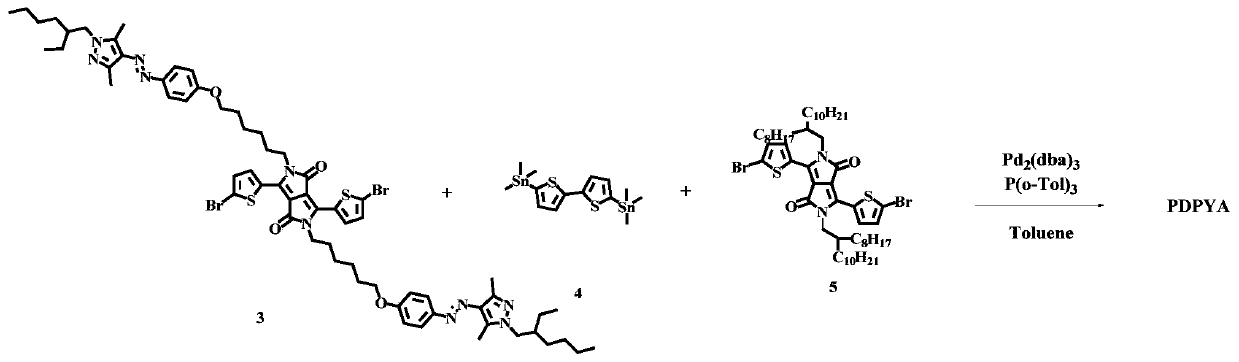
[0070] Synthesis of terpolymer shown in embodiment 2, formula I (wherein, R 1 and R 2 All are selected from 2-octyldodecyl; Ar is 2,5-thiophene substituent; when x:y=1:5, the defined polymer is PDPYA):
[0071] chemical reaction flow chart figure 2 Shown, the concrete reaction step condition of PDPYA is as follows:
[0072] 2,5-bis(2-octyldodecyl)-3,6 dibromodithiophene pyrrolopyrrole diketone (5,0.09812mmol), compound 3 obtained in Example 1 of the present invention 0.01962mmol and 2, 5-Ditrimethyltinthiophene 4 (0.1178mmol) was dissolved in anhydrous toluene, nitrogen gas was blown for 20min, catalyst tris(dibenzylideneacetone)dipalladium 0.0021mmol and ligand o-tricresylphosphine 0.017mmol were added, and continued Nitrogen was blown for 20 minutes, and under the protection of nitrogen, the reaction was carried out at 100°C for 72 hours, and then 1 mmol of trimethylphenyltin and 2 mmol of bromobenzene were added in sequence to react for 6 hours respectively for capping....
Embodiment 3
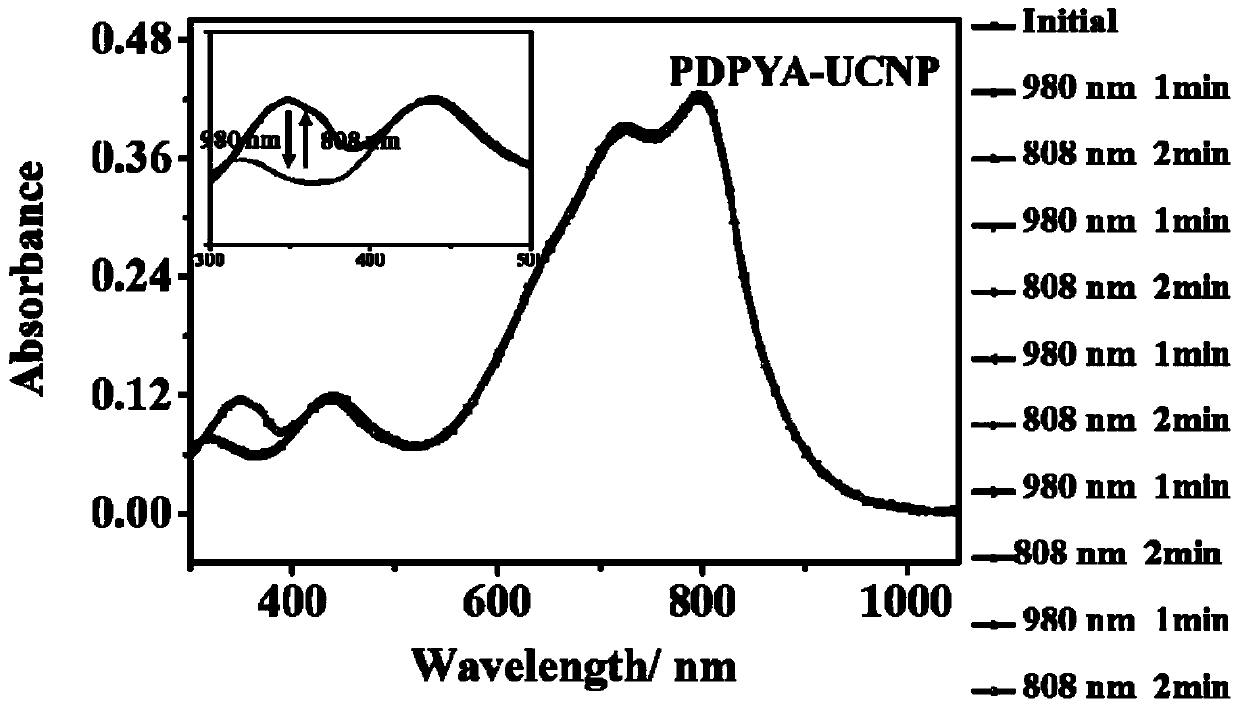
[0076] Example 3, the ultraviolet-visible absorption spectrum of the polymer described in formula I of the polymer of the present invention combined with upconversion nanoparticles under near-infrared light conditions in a thin film state:
[0077] The polymer (compound represented by formula I) prepared in Example 1-Example 2 of the present invention is dissolved in various organic solvents, organic solvents include chloroform, o-dichlorobenzene, 1,1,2,2-tetrachloro Ethane, and other solvents such as: toluene. The polymer of the present invention has good solubility in chlorinated solvents (about 20 mg / mL at room temperature). By spin-coating the o-dichlorobenzene solution of the compound shown in formula I (the drop volume is 200 microliters, the rotating speed is 3000 rpm, and the time is 1 minute) to a quartz plate to prepare a high-quality film, and then spin-coat the surface (The drop volume is 200 microliters, the rotation speed is 3000 rpm, and the time is 1 minute) U...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com