Preparation method of iron-nickel electro-catalyst and oxygen evolution application thereof under high current density
An electrocatalyst and oxygen evolution technology, applied in the field of electrocatalysis, can solve problems such as electrolysis of water conditions, harsh preparation conditions, catalyst shedding, etc., achieve excellent OER performance, improve preparation efficiency, and simplify the preparation process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
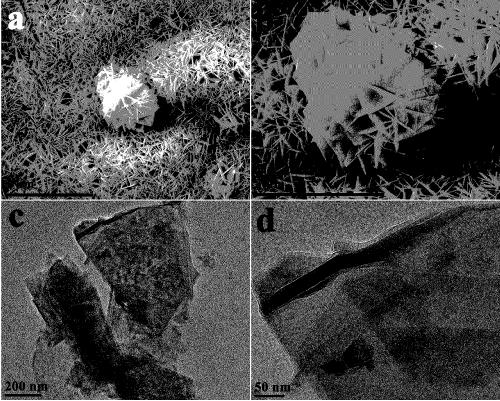
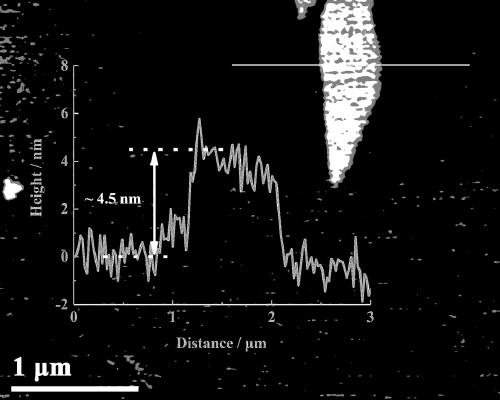
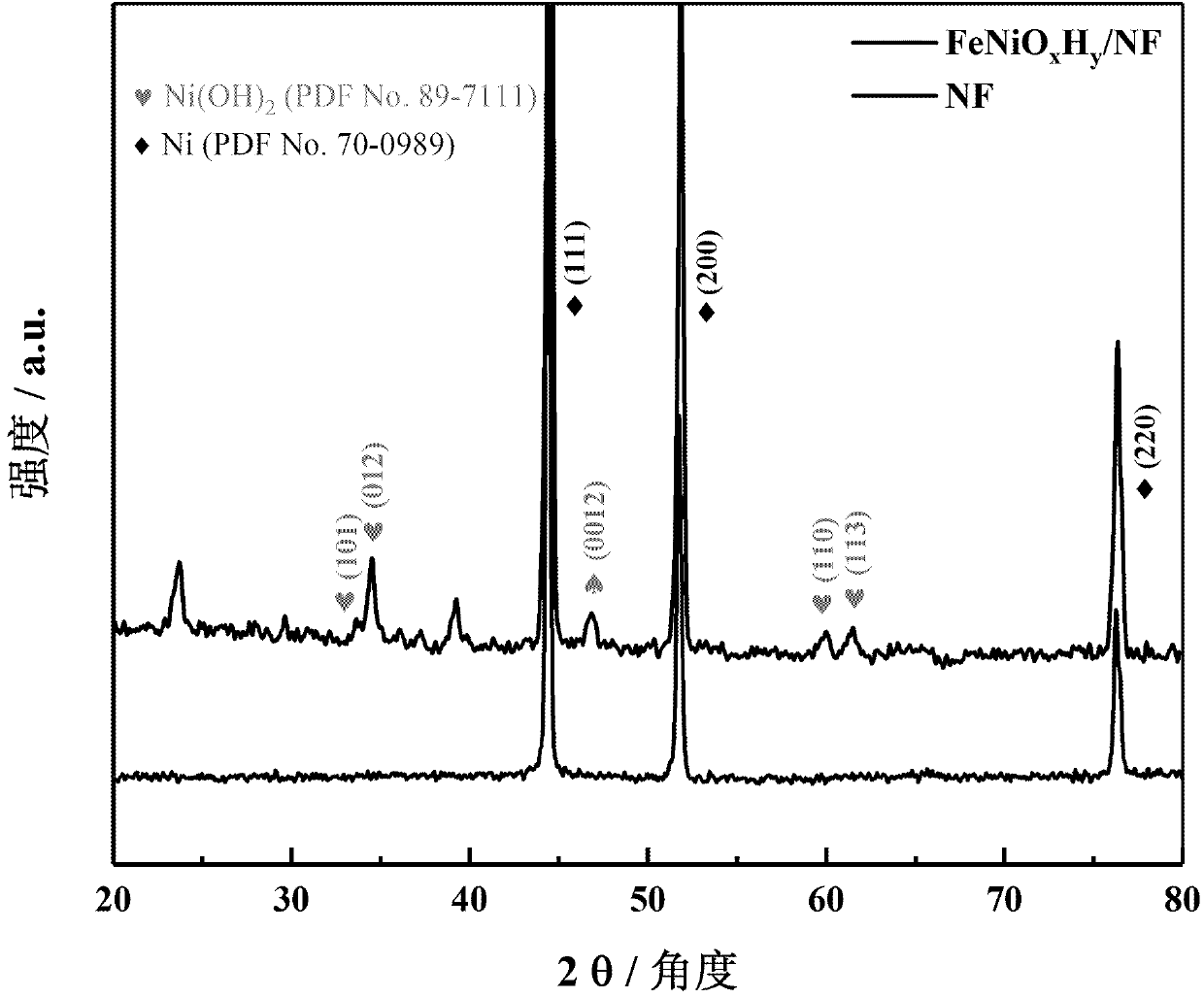
Image
Examples
Embodiment 1
[0028] (1) Use an electronic balance to accurately weigh 0.8 g of ferric nitrate (nonahydrate), 0.6 g of urea and 0.2 g of ammonium fluoride in a beaker, add 40 ml of secondary water, and mix the solution evenly under the action of a magnetic stirrer , And then use a magnet to suck out the magneton.
[0029] (2) Put the processed nickel foam (0.25 cm 2 ) Put the above homogeneous solution into a 100 ml reaction kettle for hydrothermal reaction, the reaction time of the oven is set to 1 hour, the temperature is set to 120 °C, the reaction is over and naturally cooled to room temperature, the obtained sample (FeNiO x H y / NF-1h) Rinse with water ultrasonic twice and test after drying under infrared light.
Embodiment 2
[0031] (1) Use an electronic balance to accurately weigh 0.8 g of ferric nitrate (nonahydrate), 0.6 g of urea and 0.2 g of ammonium fluoride in a beaker, add 40 ml of secondary water, and mix the solution evenly under the action of a magnetic stirrer , And then use a magnet to suck out the magneton.
[0032] (2) Put the processed nickel foam (0.25 cm 2 ) Put the above homogeneous solution into a 100 ml reaction kettle for hydrothermal reaction, the reaction time of the oven is set to 3 hours, the temperature is set to 120 °C, the reaction is over and naturally cooled to room temperature, the obtained sample (FeNiO x H y / NF-3h) Rinse clean with secondary water ultrasonic and test after drying under infrared light.
Embodiment 3
[0034] (1) Use an electronic balance to accurately weigh 0.8 g of ferric nitrate (nonahydrate), 0.6 g of urea and 0.2 g of ammonium fluoride in a beaker, add 40 ml of secondary water, and mix the solution evenly under the action of a magnetic stirrer , And then use a magnet to suck out the magneton.
[0035] (2) Put the processed nickel foam (0.25 cm 2 ) Put the above homogeneous solution into a 100 ml reaction kettle for hydrothermal reaction, the reaction time of the oven is set to 6 hours, the temperature is set to 120 °C, the reaction is over and naturally cooled to room temperature, the obtained sample (FeNiO x H y / NF-6h) Rinse with water ultrasonic twice and test after drying under infrared light.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com