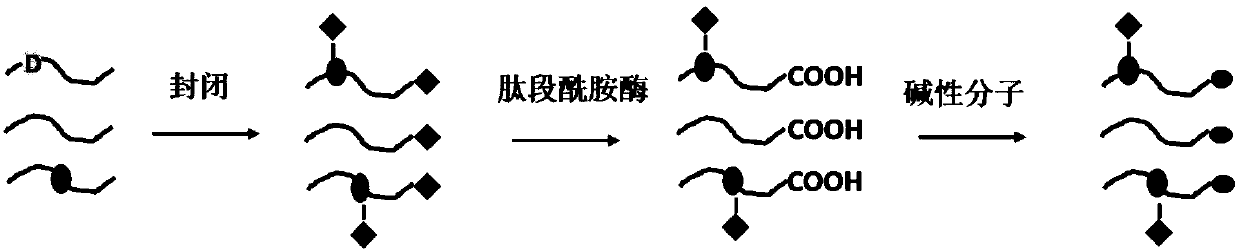
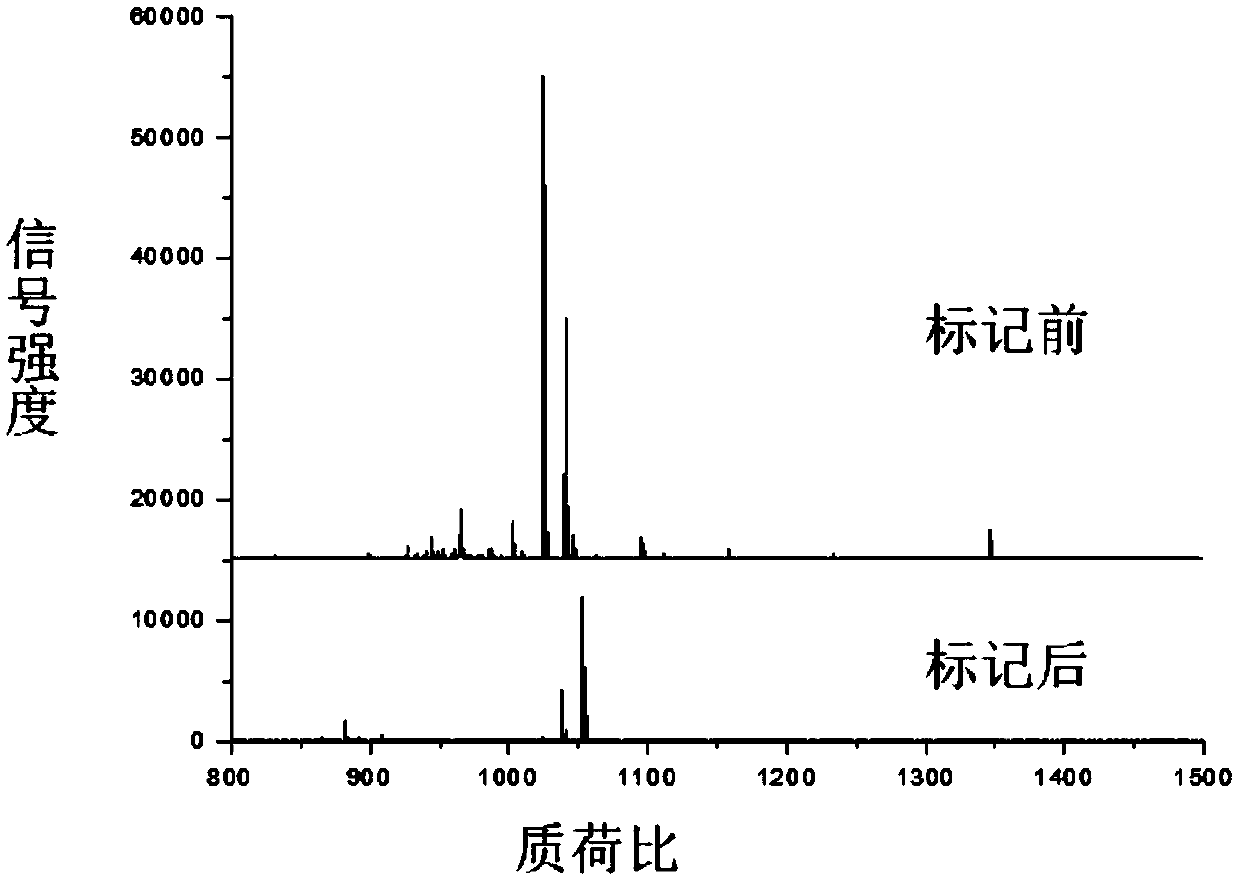
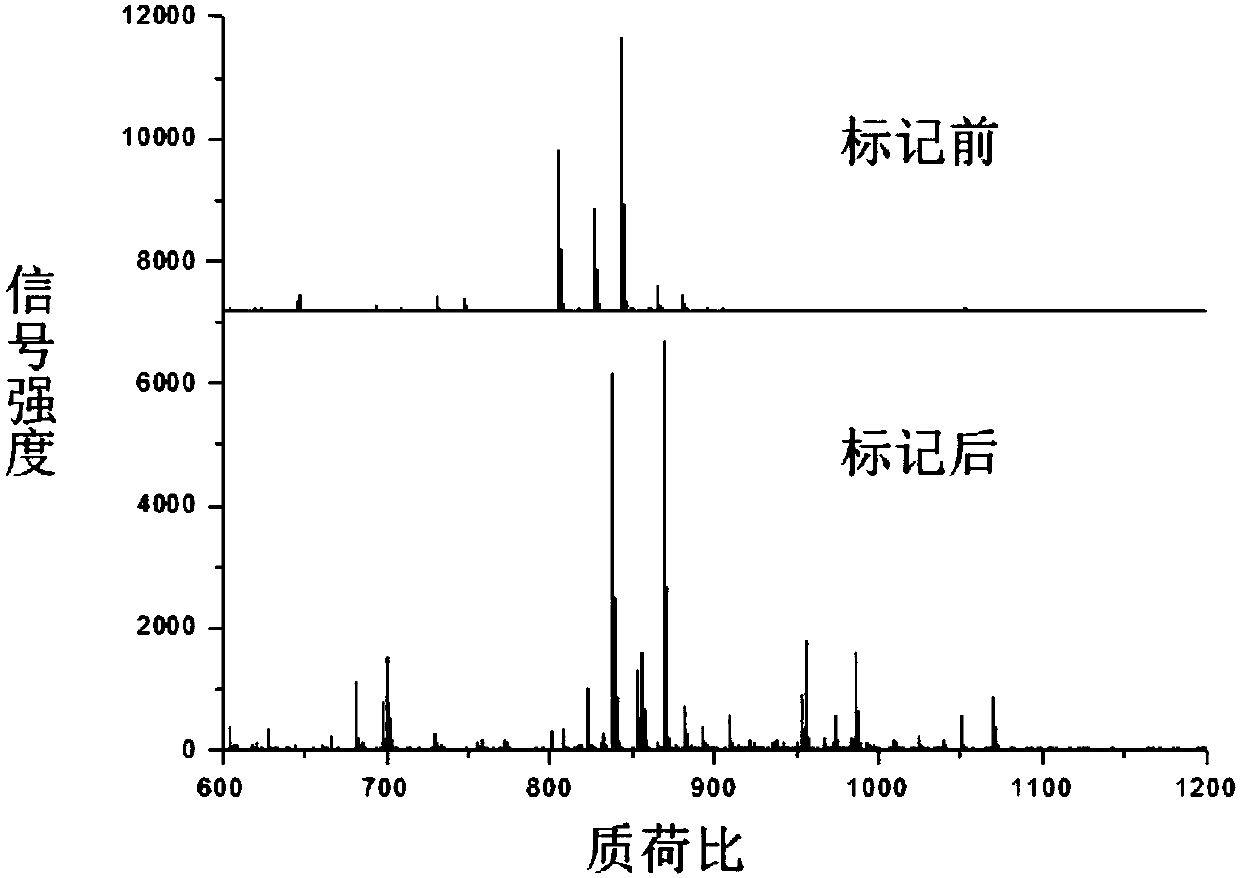
Method for improving mass spectrum fragmentation efficiency and response based on peptide fragment C-terminal chemical derivation
A peptide and chemical technology, applied in the field of improving the fragmentation efficiency and response of mass spectrometry based on the chemical derivatization of the C-terminal of the peptide, can solve the problems of unfavorable mass spectrometry identification, poor fragmentation efficiency, etc., and achieve the effect of high reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] (1) Weigh 1 mg of the synthesized model peptide CT1200 (sequence LVVSTQTALA), add 1000 μL of a mixed solution of N,N-dimethylformamide and water to make a peptide mother solution, and the volume concentration of water is 5%. Take another centrifuge tube, add 20 μL peptide master solution, 75 μL acetonitrile, 2.5 μL, 1M N-hydroxysuccinimide, 2 μL, 2.5M 1-(3-dimethylaminopropyl)-3-ethyl Carbodiimide, 0.5μL, 5M methylamine, react at 25℃ for 2h. After the reaction is completed, after freeze-drying, desalting by liquid chromatography and freeze-drying are carried out.
[0027] (2) The above product was reconstituted by adding 100 μL of tris(hydroxymethyl)aminomethane buffer salt with pH 7.5, adding 0.5 μg of peptide amidase, and hydrolyzing at 25° C. for 12 hours. After the reaction was completed, desalting was carried out by liquid chromatography and freeze-dried.
[0028] (3) The above product was redissolved by adding 93.5 μL of a mixed solution of N,N-dimethylformamide...
Embodiment 2
[0031] (1) Weigh 1 mg of the synthesized model peptide 9094 (sequence KEIFVGI), add 1000 μL of a mixed solution of N,N-dimethylformamide and water to make a peptide mother solution, and the volume concentration of water is 5%. Take another centrifuge tube, add 20 μL peptide stock solution, 74.5 μL acetonitrile, 2.5 μL, 1M N-hydroxysuccinimide, 2 μL, 2.5M 1-(3-dimethylaminopropyl)-3-ethyl Carbodiimide, 1μL, 5M methylamine, reacted at 25℃ for 2h. After the reaction is completed, after freeze-drying, desalting by liquid chromatography and freeze-drying are carried out.
[0032] (2) The above product was reconstituted by adding 100 μL of tris(hydroxymethyl)aminomethane buffer salt with pH 7.5, adding 0.5 μg of peptide amidase, and hydrolyzing at 25° C. for 12 hours. After the reaction was completed, desalting was carried out by liquid chromatography and freeze-dried.
[0033](3) The above product was redissolved by adding 90 μL of a mixed solution of N,N-dimethylformamide and wa...
Embodiment 3
[0036] (1) Weigh 1 mg of the purchased drug peptide oxytocin (sequence CYIQNCPLG), add 1000 μL of acetone and water mixed solution to make peptide mother solution, and the volume concentration of water is 5%. Take another centrifuge tube, add 20 μL of peptide stock solution, 65.5 μL of N,N-dimethylformamide, 2.5 μL of 1M N-hydroxysuccinimide, 2 μL of 2.5M 1-(3-dimethylformamide Aminopropyl)-3-ethylcarbodiimide, 10 μL, 5M ethanolamine, reacted at 25°C for 2h. After the reaction is completed, after freeze-drying, desalting by liquid chromatography and freeze-drying are carried out.
[0037] (2) The above product was reconstituted by adding 100 μL of tris(hydroxymethyl)aminomethane buffer salt with pH 7.5, adding 0.1 μg of peptide amidase, and hydrolyzing at 30° C. for 24 hours. After the reaction was completed, desalting was carried out by liquid chromatography and freeze-dried.
[0038] (3) The above product was redissolved by adding 93.5 μL of a mixed solution of N,N-dimethy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com