Preparation and application of sugar-targeted modified siRNA nanoparticle
A nanoparticle and targeted modification technology, applied in DNA/RNA fragments, recombinant DNA technology, medical preparations of non-active ingredients, etc., can solve the problem that it is difficult for drugs to reach the lesion effectively, Alzheimer's is difficult to reach, The treatment effect is unsatisfactory and other problems, to achieve the effect of promoting blood circulation time, increasing effective accumulation, and good half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
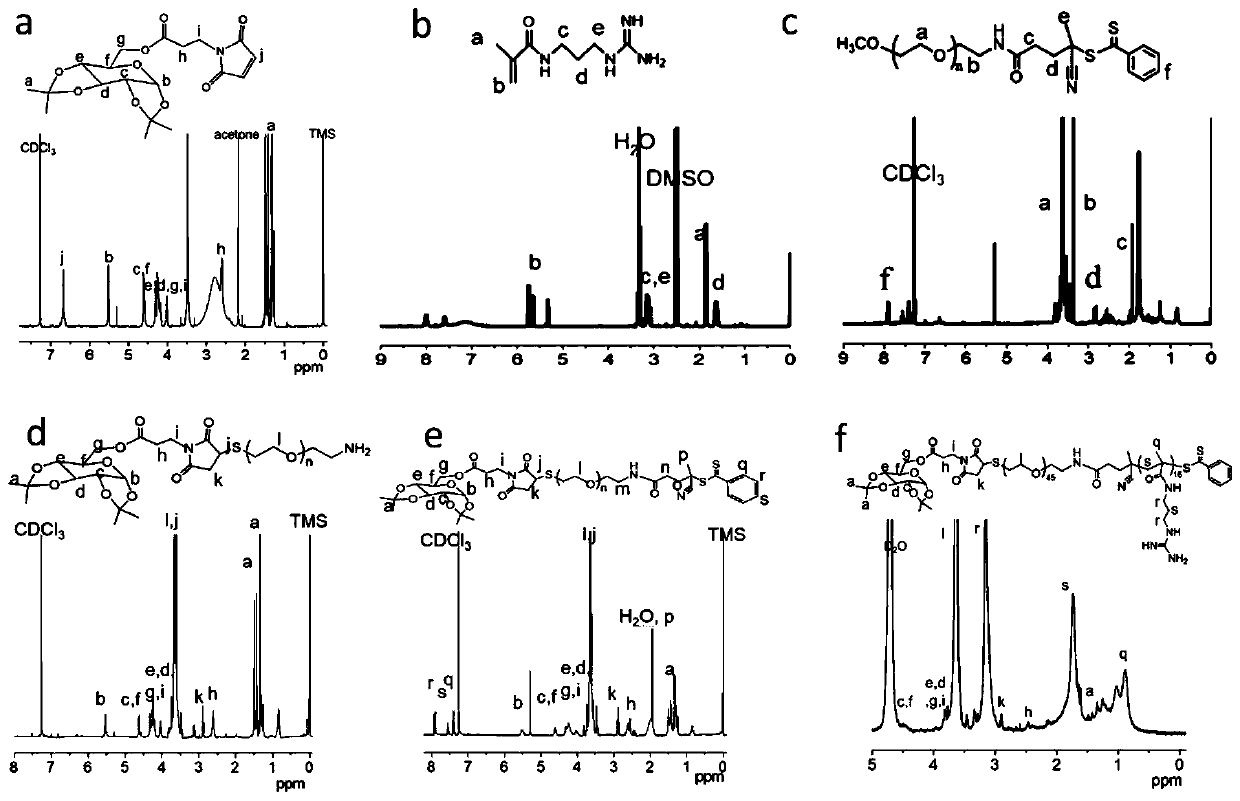
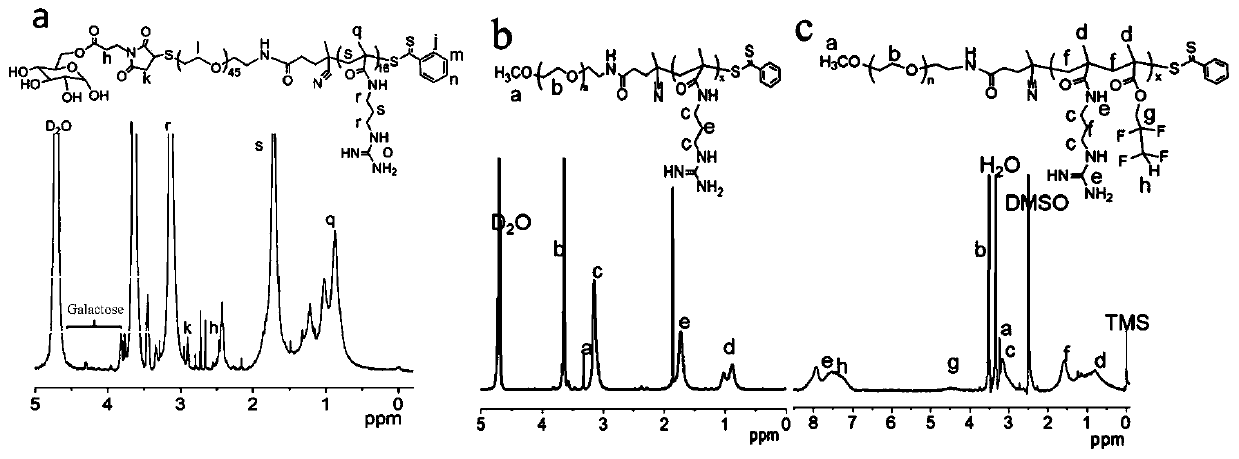
[0070] This embodiment provides a preparation method of targeting nanocarriers, which specifically includes the following organic synthesis steps. The targeting molecule in this example is galactose (Gal), the first linking compound is maleimidopropionic acid, the second linking compound is CPADB, and the first hydrophilic biomaterial is HS-PEG-NH 2 .
[0071] The structural formulas of dGal and dGal-Mal are as follows:
[0072]
[0073] Maleimide was grafted onto galactose through the esterification reaction of carboxyl and hydroxyl groups. The specific reaction steps were as follows: 3-maleimidopropionic acid (0.50g, 2.96mmol) and 1,2,3 , 4-di-O-isopropylidene-D-α-galactopyranose (0.82g, 3.15mmol) was dissolved in 12mL pyridine / dichloromethane solution (1 / 1=v / v), added N-ethyl-N'-(3-(dimethylamino)propyl)carbodiimide hydrochloride (EDC HCl) (0.695g, 3.62mmol), N,N-lutidine-4 - Amine (DMAP) (19 mg, 0.15 mmol), the reaction mixture was stirred at room temperature for 24 ...
Embodiment 2
[0096] This example provides a preparation method of non-targeting nanocarriers (MeO-PEG-PGUA and MeO-PEG-PGUAF), which specifically includes the following organic synthesis steps. The second connection compound in this example is CPADB, and the second hydrophilic biomaterial is MeO-PEG-NH 2 .
[0097] (1) Synthesis of MeO-PEG-CPADB.
[0098] Under ice bath conditions, CPADB (230.4mg, 0.696mmol) and NHS (100.8mg, 0.852mmol) were dissolved in 43.8mL DCM, and the DCM solution of EDC (408.6mg, 1.986mmol dissolved in 8.766mL DCM) The ice bath was removed one hour after the end of the dropwise addition. The reaction mixture was stirred at room temperature for 20 h, MeO-PEG-NH 2 (464 mg, 0.232 mmol), the reaction was allowed to stir at room temperature for 8 hours. MeO-PEG-CPADB was obtained by precipitating in cold ether, centrifuging (8000rpm, 5min, 4°C), collecting the precipitate, and drying in vacuo. The equation of the synthesis reaction is as follows:
[0099]
[010...
Embodiment 3
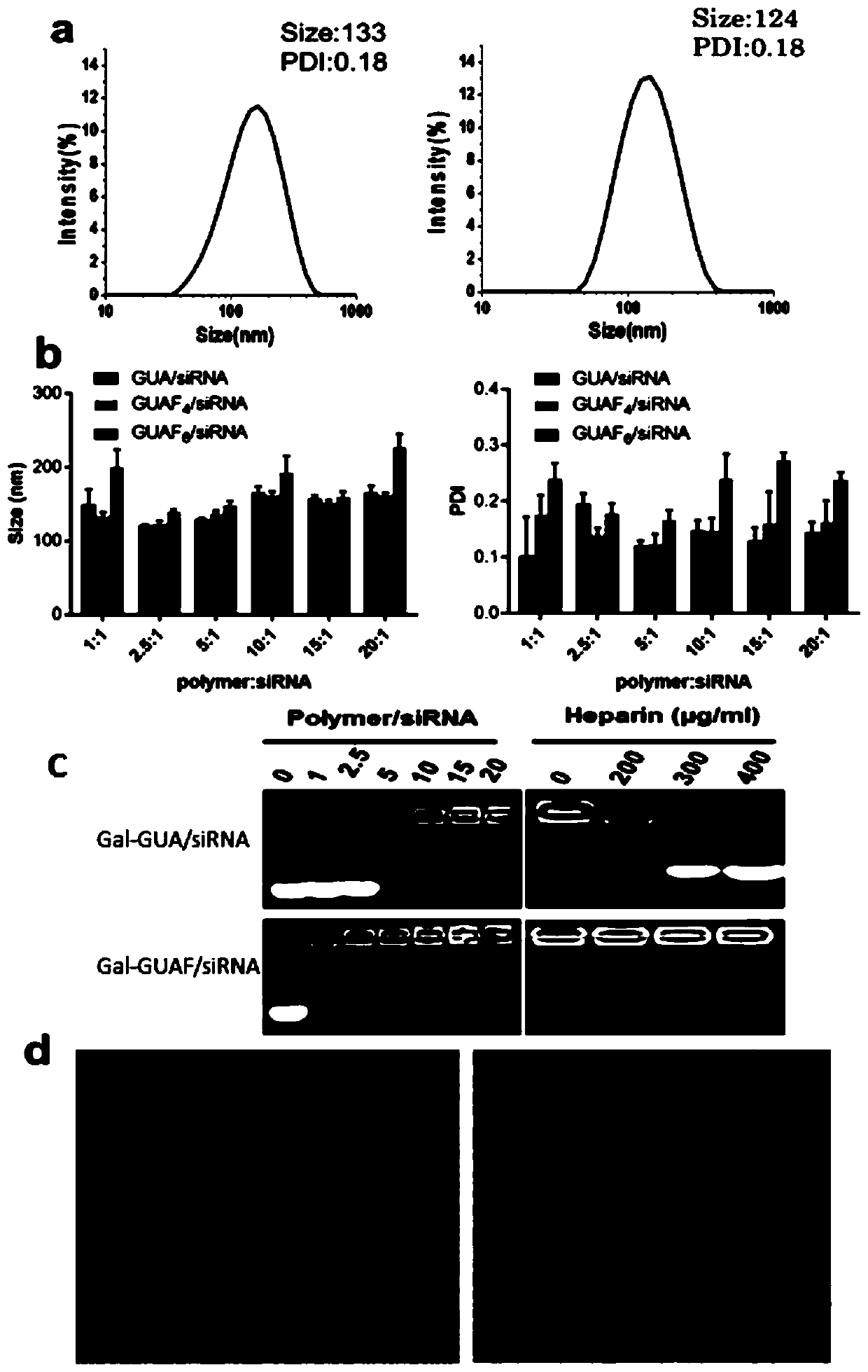
[0109] This example provides a method for preparing sugar-targeting modified siRNA nanoparticles.
[0110] First, centrifuge the dry siRNA powder (7000 rpm, 4 min) to precipitate the siRNA, then add 500 μL of DEPC water to prepare a 1 mg / mL siRNA solution.
[0111] Then, the polymer (1 mg, including the targeted nanocarrier prepared in Example 1 and the non-targeted fluorine-containing nanocarrier MeO-PEG-PGUAF prepared in Example 2 was dissolved in 10 mM Hepes buffer solution (pH=7.4, 10 mg / mL ), wherein the molar ratio of targeted nanocarriers and non-targeted carriers was 1:3, siRNA (15 μM in 10 mM HEPES buffer) was added and incubated at room temperature for 1 hour. Sugar-targeted nanoparticles were polymerized by mass ratio: siRNA=2.5:1 was prepared.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com