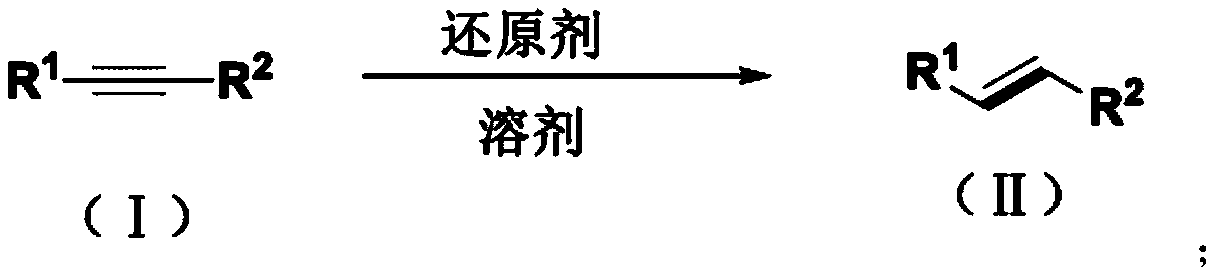
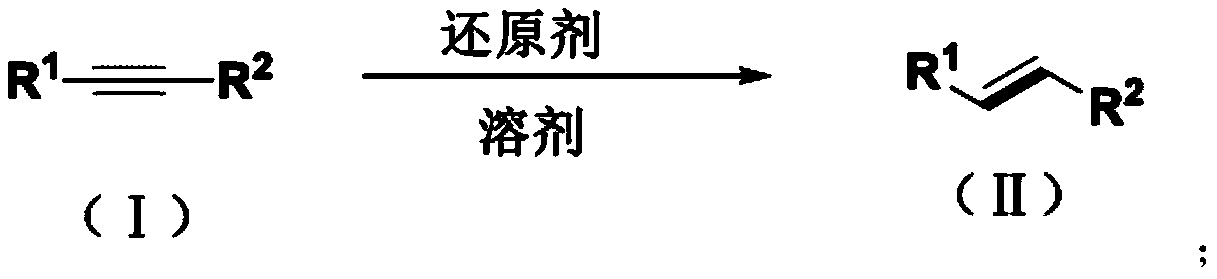
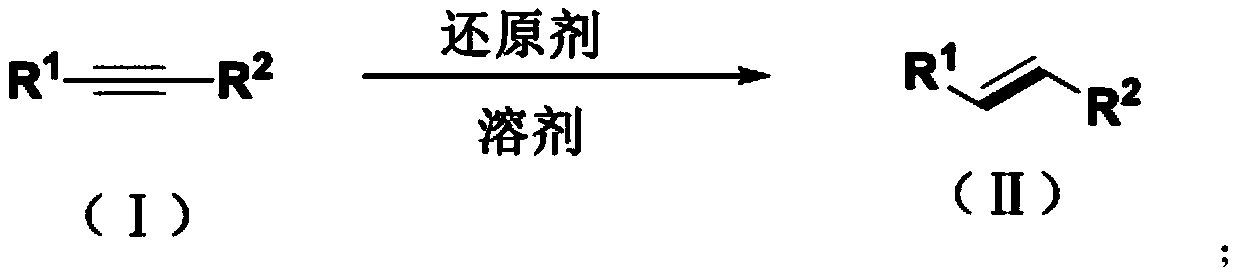
Method for synthesizing trans-olefin compound
A synthesis method and technology for alkyne compounds, applied in the field of olefin compound synthesis, can solve problems such as reaction conditions impairing functional group tolerance, few reports, and exploration of unalkylated alkynes, etc., achieving excellent biological activity and reducing pollution , the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1: the synthesis of (E)-1,2-stilbene
[0043]
[0044]a): Take a 25 ml reaction tube, add 178 mg of tolan, 152 mg of thioacetamide, 36 microliters of water, 2 ml of N,N-dimethylformamide, and stir for 12 hours at 130° C. After the reaction, add 10 ml of ethyl acetate to quench the reaction, add 10 ml of saturated brine to wash, separate the organic phase, extract the water phase with ethyl acetate three times (10 ml*3), combine the organic phases, add anhydrous sodium sulfate to dry , the solvent was distilled off under reduced pressure, and then separated by column chromatography to obtain 28 mg of (E)-1,2-stilbene with a yield of 16%.
[0045] b): Take a 25 ml reaction tube, add 178 mg of but-1-yn 1-ylbenzene, 332 mg of N,N-dimethylammonium dithiocarbamate, 36 microliters of water, N, 2 ml of N-dimethylformamide, stirred and reacted at 130°C for 12 hours, after the reaction was completed, 10 ml of ethyl acetate was added to quench the reaction, and 10 m...
Embodiment 2
[0055] Embodiment 2: the synthesis of (E)-(2-cyclohexyl vinyl) benzene
[0056]
[0057] Take a 25ml reaction tube, add 184mg of cyclohexylethynylbenzene, 320mg of potassium ethyl xanthate, 36μl of water, 2ml of N,N-dimethylformamide, and stir at 130°C for 12 hours After the reaction, add 10 ml of ethyl acetate to quench the reaction, add 10 ml of saturated brine to wash, separate the organic phase, and extract the aqueous phase with ethyl acetate for 3 times (10 ml*3) to combine the organic phases, add anhydrous sodium sulfate After drying, the solvent was distilled off under reduced pressure, and then separated by column chromatography to obtain 132 mg of (E)-(2-cyclohexylvinyl)benzene with a yield of 72%.
[0058] 1 H NMR (400MHz, CDCl 3 )δ7.36(d, J=7.6Hz, 2H), 7.29(dd, J=15.2, 7.2Hz, 2H), 7.20(t, J=7.2Hz, 1H), 6.36(d, J=16.0Hz, 1H), 6.20(dd, J=15.6, 6.8Hz, 1H), 2.18–2.10(m, 1H), 1.78(dd, J=14.4, 11.2Hz, 4H), 1.70(d, J=12.8Hz, 1H ),1.37–1.29(m,2H),1.26–1.16(m,3H); 1...
Embodiment 3
[0059] Embodiment 3: the synthesis of (E)-(3,3-dimethylbut-1-en-1-yl)benzene
[0060]
[0061] Take a 25 ml reaction tube, add (3,3-dimethylbut-1-yn-1-yl)benzene 158 mg, ethyl xanthate potassium 320 mg, water 36 microliters, N,N-di Methylformamide 2 ml, stirred at 130°C for 12 hours, after the reaction was completed, 10 ml of ethyl acetate was added to quench the reaction, and 10 ml of saturated brine was added for washing, the organic phase was separated, and the aqueous phase was extracted 3 times with ethyl acetate (10ml*3) Combine the organic phases, add anhydrous sodium sulfate to dry, remove the solvent by distillation under reduced pressure, and then separate by column chromatography to obtain (E)-(3,3-dimethylbut-1-ene-1- Base) 66 mg of benzene, the yield is 42%.
[0062] 1 H NMR (600MHz, CDCl 3 )δ7.38(d, J=7.2Hz, 2H), 7.31(dd, J=7.2, 1.8Hz, 2H), 7.20(t, J=7.2Hz, 1H), 6.32(d, J=16.2Hz, 1H), 6.27(d, J=16.2Hz, 1H), 1.14(s, 9H); 13 C NMR (150MHz, CDCl 3 )δ141.8, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com