A kind of preparation method of bacitracin impurity 1 based on photocatalysis
A bacitracin and photocatalytic technology, which is applied in the field of biochemistry, can solve the problems of low bacitracin impurity L content, unsuitable preparation method, and low preparation efficiency, and achieve the effects of simple post-processing, low cost, and simple steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
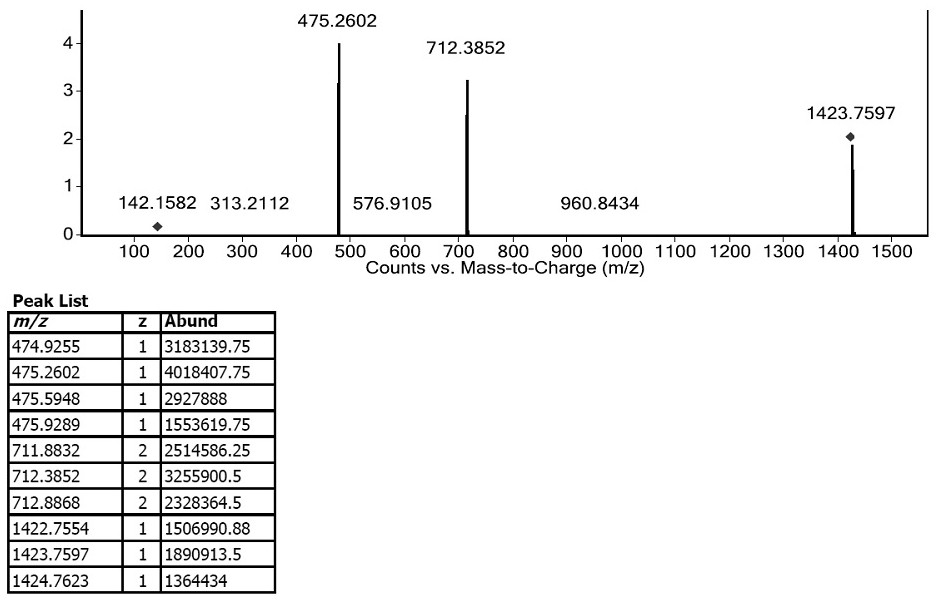
[0027] Instruments: Preparative Liquid Phase (Waters 2525); Rotary Rotary Evaporator (Shanghai Yarong); Photochemical Reactor (Shanghai Julai Experimental Instrument Co., Ltd.).
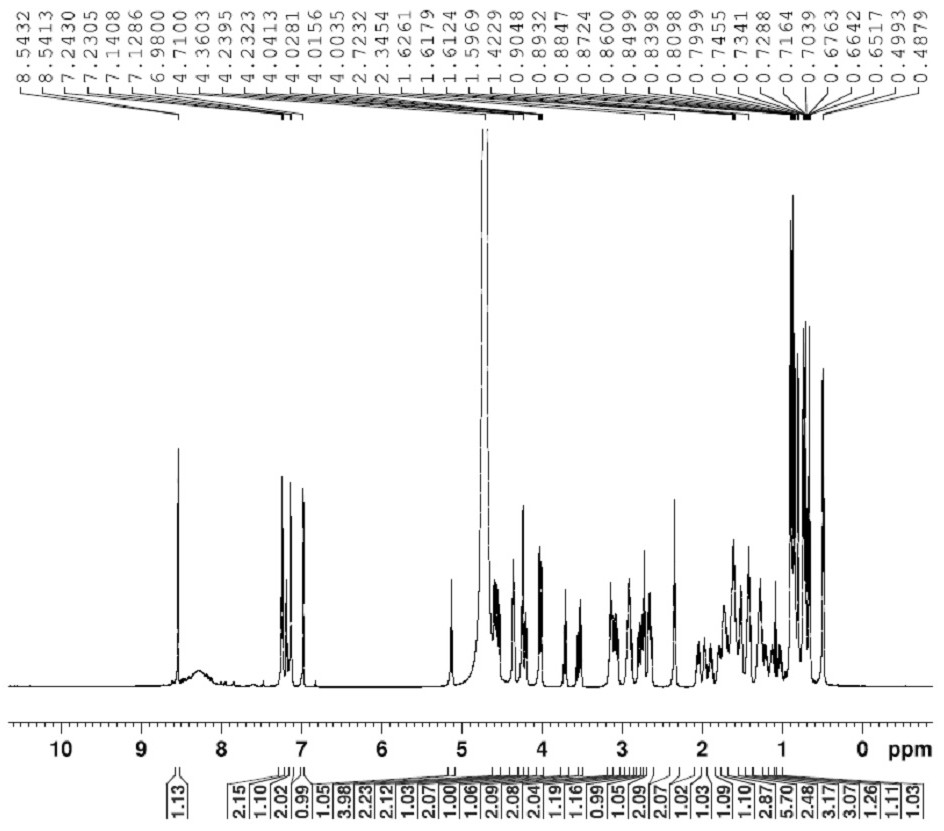
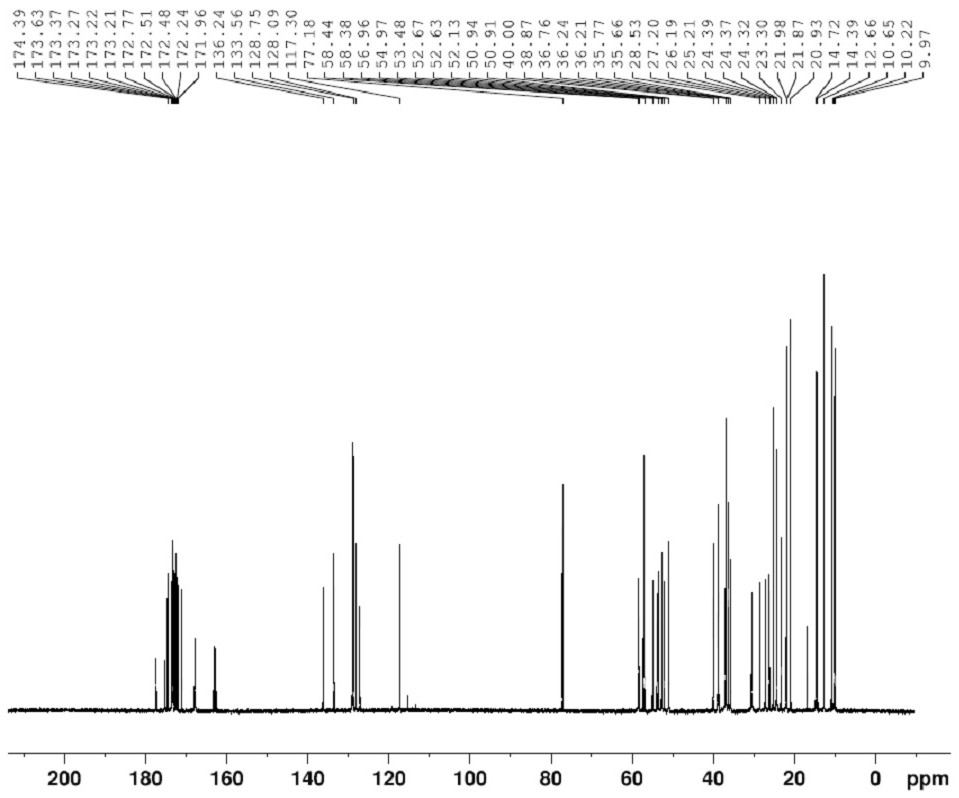
[0028] Preparation of crude bacitracin impurity L: Dissolve 3 g of bacitracin raw material in 50 ml of ethanol, add 1 ml of trifluoroacetic acid to aid dissolution, and react for 2 hours at 50° C. with a light intensity of 5000 Lux in a photochemical reactor to obtain a content of impurity L of 30%. crude products. The above content is the content characterized by the HPLC area normalization method.
[0029] Preparative column: the filler is Kromasil EternityXT-10-C18, and the column tube is HB-DAC50.
[0030] Preparative liquid phase separation chromatographic conditions: carry out isocratic elution with 0.1% volume concentration trifluoroacetic acid aqueous solution-acetonitrile (70:30, V / V) as mobile phase; Flow rate is 100mL / min; Detection wavelength 254nm; Column temperature is Room temperatur...
Embodiment 2
[0046] Instruments: Preparative Liquid Phase (Waters 2525); Rotary Rotary Evaporator (Shanghai Yarong); Photochemical Reactor (Shanghai Julai Experimental Instrument Co., Ltd.).
[0047] Preparation of crude bacitracin impurity L: Dissolve 3 g of bacitracin raw material in 30 ml of methanol, add 1 ml of trifluoroacetic acid, and react at 50° C. for 3 hours in a photochemical reactor with light intensity of 4500 Lux to obtain a crude product with an impurity L content of 35%. . The above content is the content characterized by the HPLC area normalization method.
[0048] Preparative column: the filler is Kromasil EternityXT-10-C18, and the column tube is HB-DAC50.
[0049] Preparative liquid phase separation chromatographic conditions: carry out isocratic elution with 0.1% volume concentration trifluoroacetic acid aqueous solution-acetonitrile (70:30, V / V) as mobile phase; Flow rate is 100mL / min; Detection wavelength 254nm; Column temperature is Room temperature; manual injec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com