A kind of analysis method of fosfomycin tromethamine genotoxic impurity
A technology of fosfomycin trometamol and analysis method, which is applied in the direction of analysis of materials, material separation, and resistance to vector-borne diseases, etc., can solve problems such as failure to meet detection limit requirements, and achieve low cost, strong specificity, and high The effect of sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1. a kind of analytical method of fosfomycin tromethamine genotoxic impurity
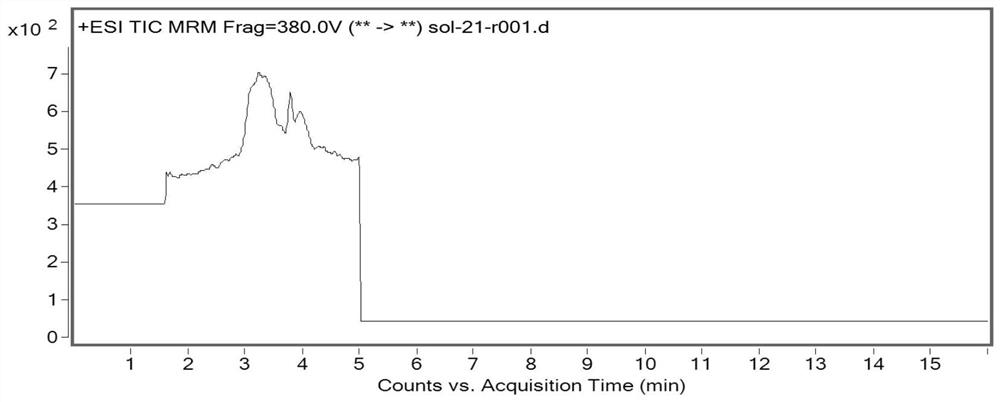
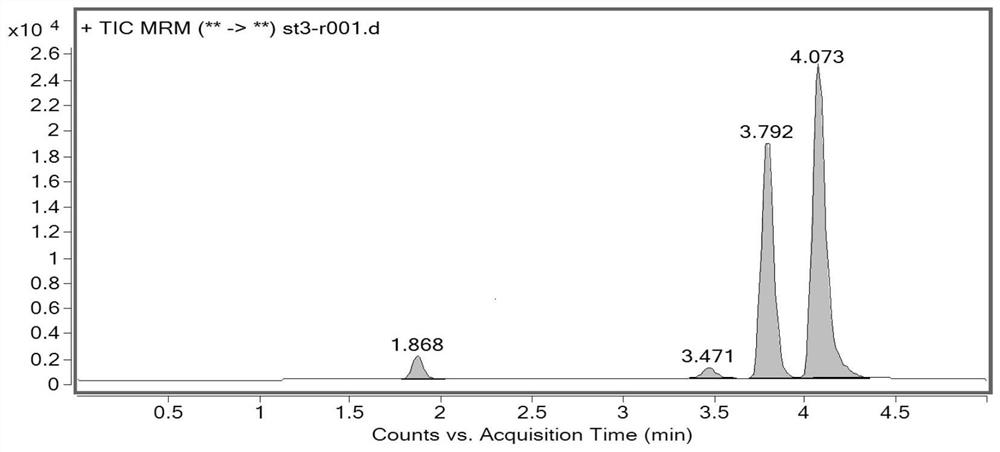
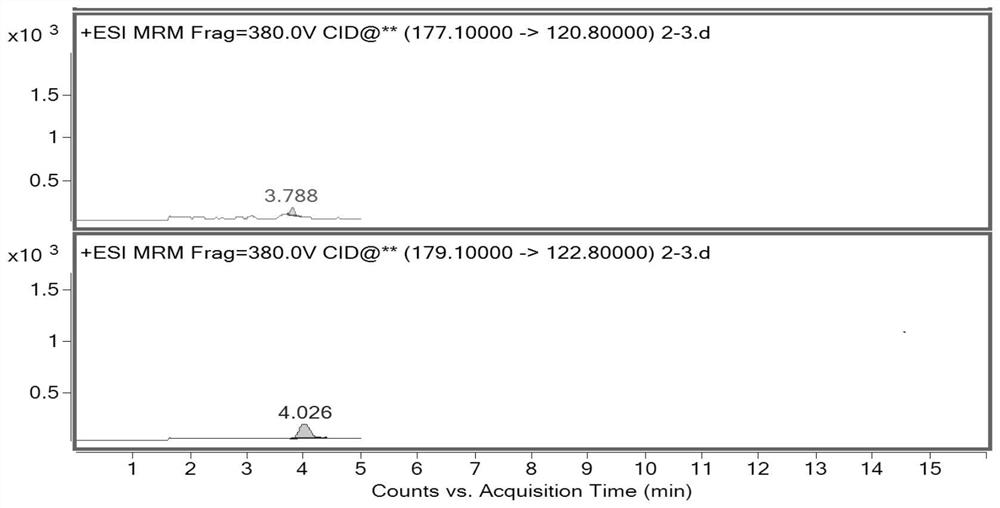
[0035]This embodiment mainly describes a method for analyzing the genotoxic impurities of fosfomycin tromethamine, and the genotoxic impurities include: allene diethyl phosphate (impurity A), 1-propene diethyl phosphate ( Impurity B), 3-diethyl phosphate-3-methyl-propylene oxide (impurity C), or dimethyl 2-phosphate-3-methyl-propylene oxide (impurity D).
[0036] 1. Reference substance
[0037] Diethyl allene phosphate (impurity A): batch number: 20190408, content: 94.78 %.
[0038] 1-Propylene diethyl phosphate (impurity B): batch number: 20190409, content: 98.79%.
[0039] 3-Diethyl phosphate-3 methyl-propylene oxide (impurity C): batch number: 20190916, content: 94.34%.
[0040] Dimethyl 2-phosphate-3-methyl-propylene oxide (impurity D): batch number: 20190918, content: 94.61%.
[0041] 2. Experimental method
[0042] 2.1 Method
[0043] Take 80 mg of fosfomycin tromethami...
Embodiment 2
[0061] Embodiment 2. a kind of analytical method of fosfomycin tromethamine genotoxic impurity
[0062] The present embodiment mainly describes a kind of analytical method of fosfomycin tromethamine genotoxicity impurity, the difference with embodiment 1 lies in the following technical parameters:
[0063] (1) Mobile phase A is an aqueous solution containing 10% formic acid;
[0064] (2) The test solution was prepared by the following method: take 80 mg of fosfomycin tromethamine crude drug, add it into an aqueous solution containing 95% methanol, vortex to dissolve, shake well, and use it as the test solution.
[0065] (3) The ratio of the value of the weight (mg) of the fosfomycin tromethamine bulk drug to the value of the volume (mL) of the test solution is 90:1;
[0066] (4) The rate of the gradient elution is 0.85mL / min, and the temperature of the eluted chromatographic column is 32°C;
[0067] (5) The high performance liquid chromatography-mass spectrometry method incl...
Embodiment 3
[0069] Embodiment 3. a kind of analytical method of fosfomycin tromethamine genotoxic impurity
[0070] The present embodiment mainly describes a kind of analytical method of fosfomycin tromethamine genotoxicity impurity, the difference with embodiment 2 lies in the following technical parameters:
[0071] (1) Mobile phase A is an aqueous solution containing 1% formic acid;
[0072] (2) The test solution is prepared by the following method: take 80 mg of fosfomycin tromethamine API, add it into an aqueous solution containing 85% methanol, vortex to dissolve, shake well, and use it as the test solution.
[0073] (3) The ratio of the value of the weight (mg) of the fosfomycin tromethamine bulk drug to the value of the volume (mL) of the test solution is 70:1;
[0074] (4) The rate of the gradient elution is 0.75 mL / min, and the temperature of the eluted chromatographic column is 28°C.
[0075] After setting the above technical parameters, the inventor obtained the same results...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| percent by volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com