High-efficiency cyhalothrin hapten, activated carrier protein, complete antigen and polyclonal antibody for immunoassay
A high-efficiency cyhalothrin and immunoassay technology, applied in the direction of immunoglobulin, immunoglobulin from serum, ovalbumin, etc., can solve the problems of non-specificity, low total yield, high toxicity, etc., and achieve strong specificity The effect of stability, easy industrial production, and high structural fidelity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
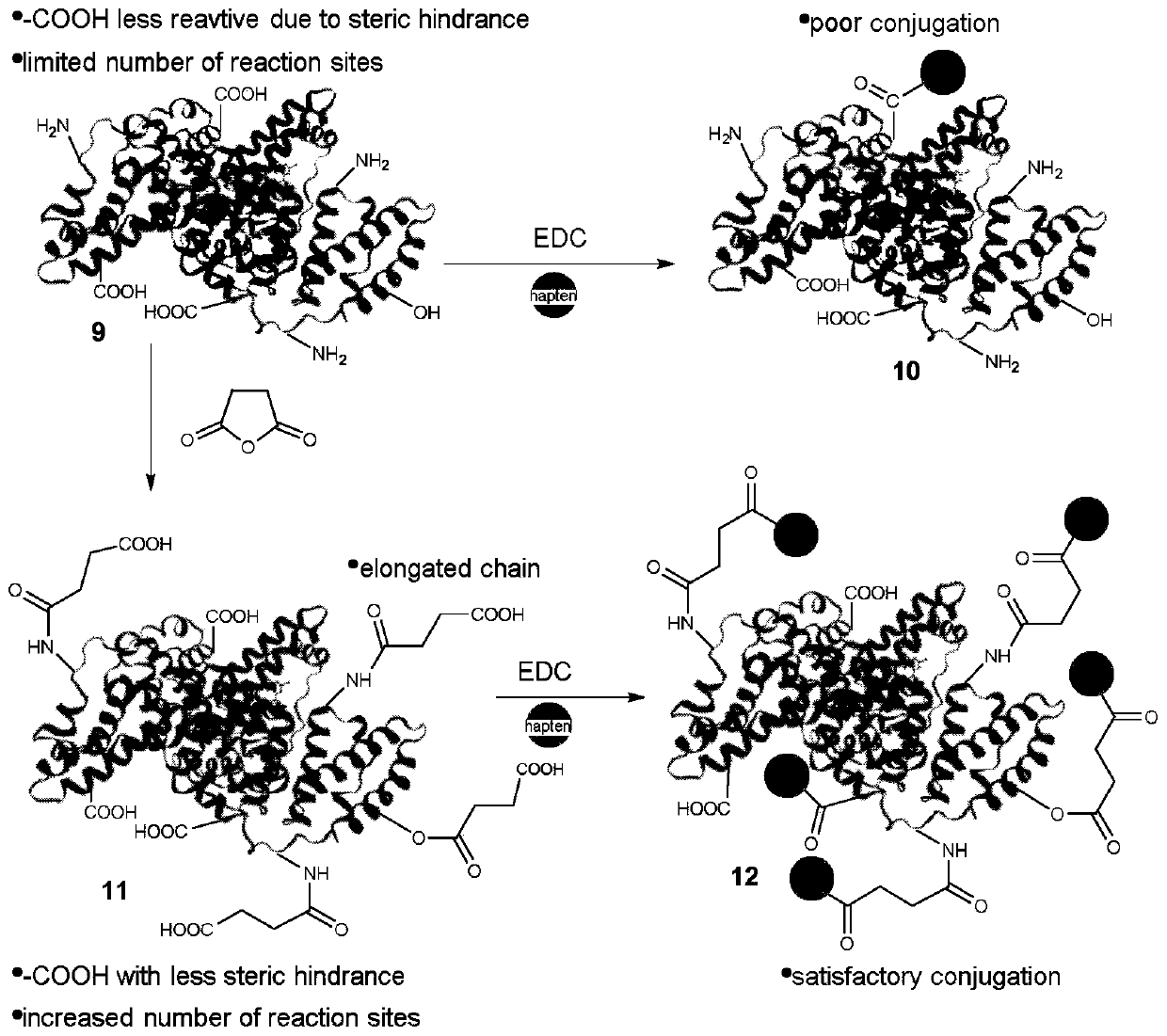
[0050] The raw material is in mmol, and the one-step synthesis method specifically includes the following steps: 1.5-2.5 mmol of efficient-cyhalothrin is packed into a reaction vessel and cooled in an ice-water bath, and then 3.5-4.5 mmol of trimethyl ((trifluoromethyl )sulfonyl)silane, stirred for 1-3min until uniform; after lambda-cyhalothrin is completely dissolved, slowly add 70-80μL of water within 1.5-2.5min and stir the resulting mixture for 3-5h; then use saturated NaHCO 3 solution to neutralize the reaction, the organic reagent CH 2 Cl 2 Extract multiple times, each time with an amount of 10-20mL; mix the organic phases and use excess Na 2 SO 4 Dry and concentrate in vacuo; the product is separated using a silica gel column, and the eluent is a mixture of ethyl acetate and n-hexane with a volume ratio of (35-65):(45-55).
[0051] The preparation method of lambda-cyhalothrin complete antigen comprises the following steps:
[0052] 1) carrier protein activation: th...
specific Embodiment
[0057] Material
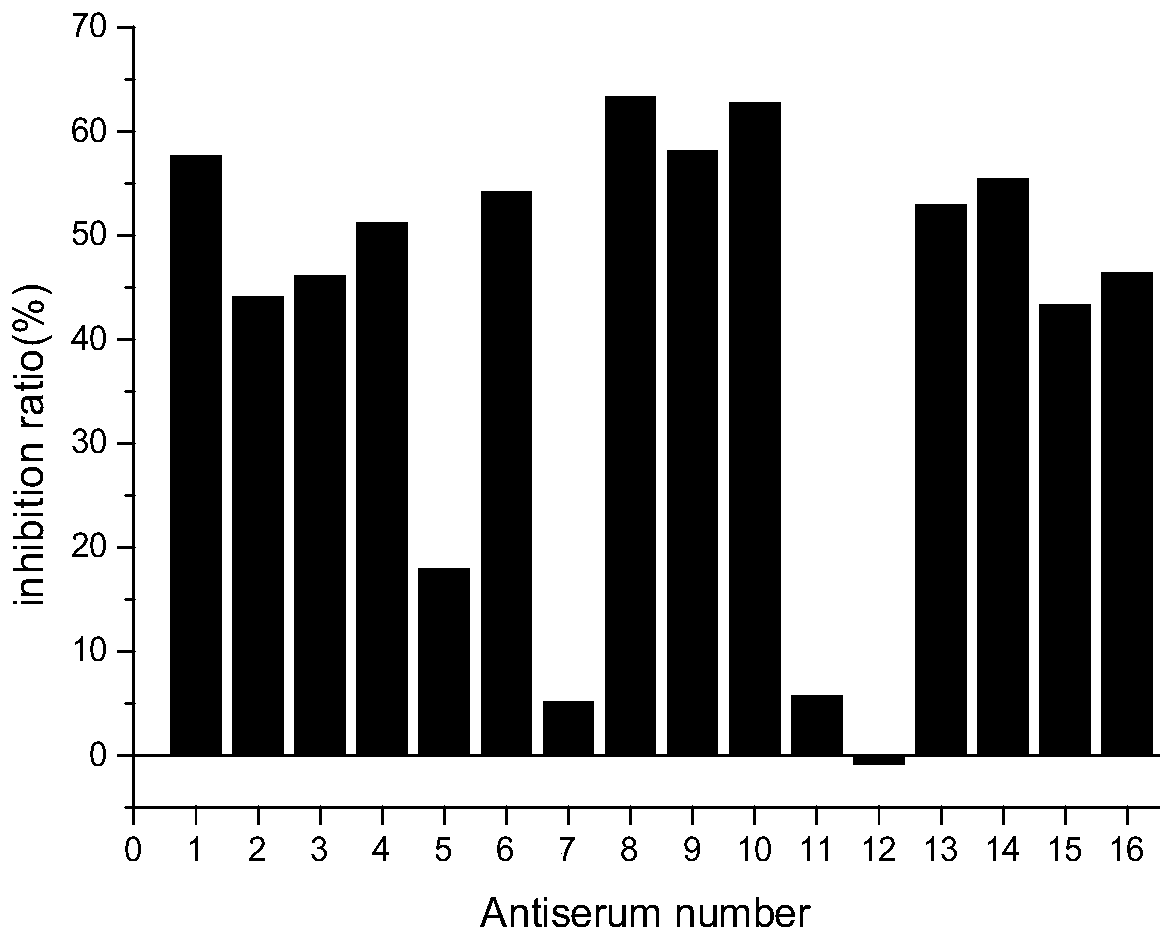
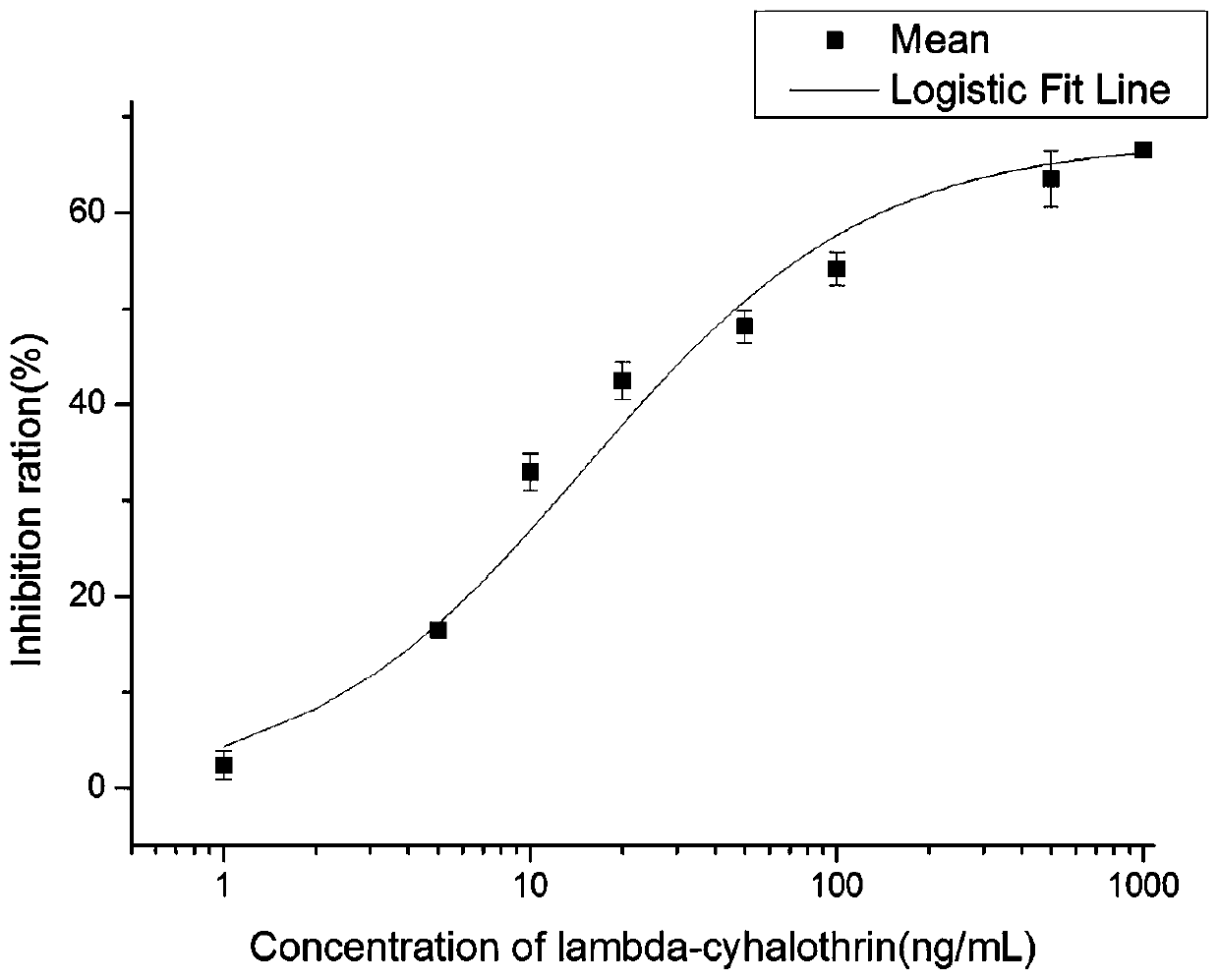
[0058] Reagents: silica gel (125-150 μm) was purchased from Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China); lambda-cyhalothrin was purchased from Wuhan Yuancheng Gongchao Technology Co., Ltd. (Wuhan, China); Deltamethrin, cypermethrin, cyfluthrin, and esfenvalerate were all obtained from the National Research Center for Standard Materials of China (Beijing, China). Skim milk powder was purchased from Reanal (Hungary). Chemicals for Hapten Synthesis and Conjugation to Carrier Proteins, Horseradish Peroxidase (HRP), Bovine Serum Albumin (BSA), Ovalbumin (OVA), and Tetramethylbenzidine (TMB) Conjugated Goat Anti-mouse IgG was purchased from Sigma-Aldrich Chemical Co. (Milwaukee, USA). α-cyano-4 hydroxycinnamic-acid (HCCA), trifluoroacetic acid (TFA) were purchased from Sigma-Aldrich Chemical Co., Ltd. (Milwaukee, USA).
[0059] Apparatus: Enzyme-linked immunosorbent assays (ELISAs) were performed in 96-well microplates (No. 3655) purchase...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com