Preparation method of hydroxamic acid
A technology of hydroxamic acid and acid anhydride, which is applied in the field of preparation of hydroxamic acid, can solve the problems of limiting the popularization and application of hydroxamic acid, low product conversion rate, hazardous waste of chemical by-products, etc., and achieves reduction in consumption of alkali and reduction in consumption. Reduced, highly reactive effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
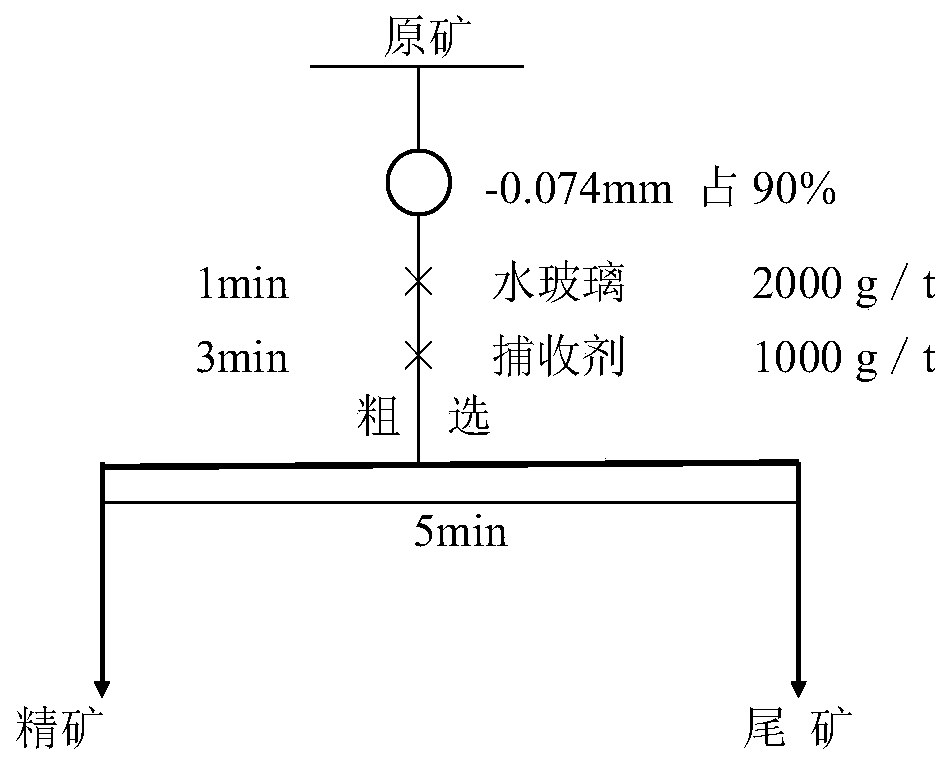
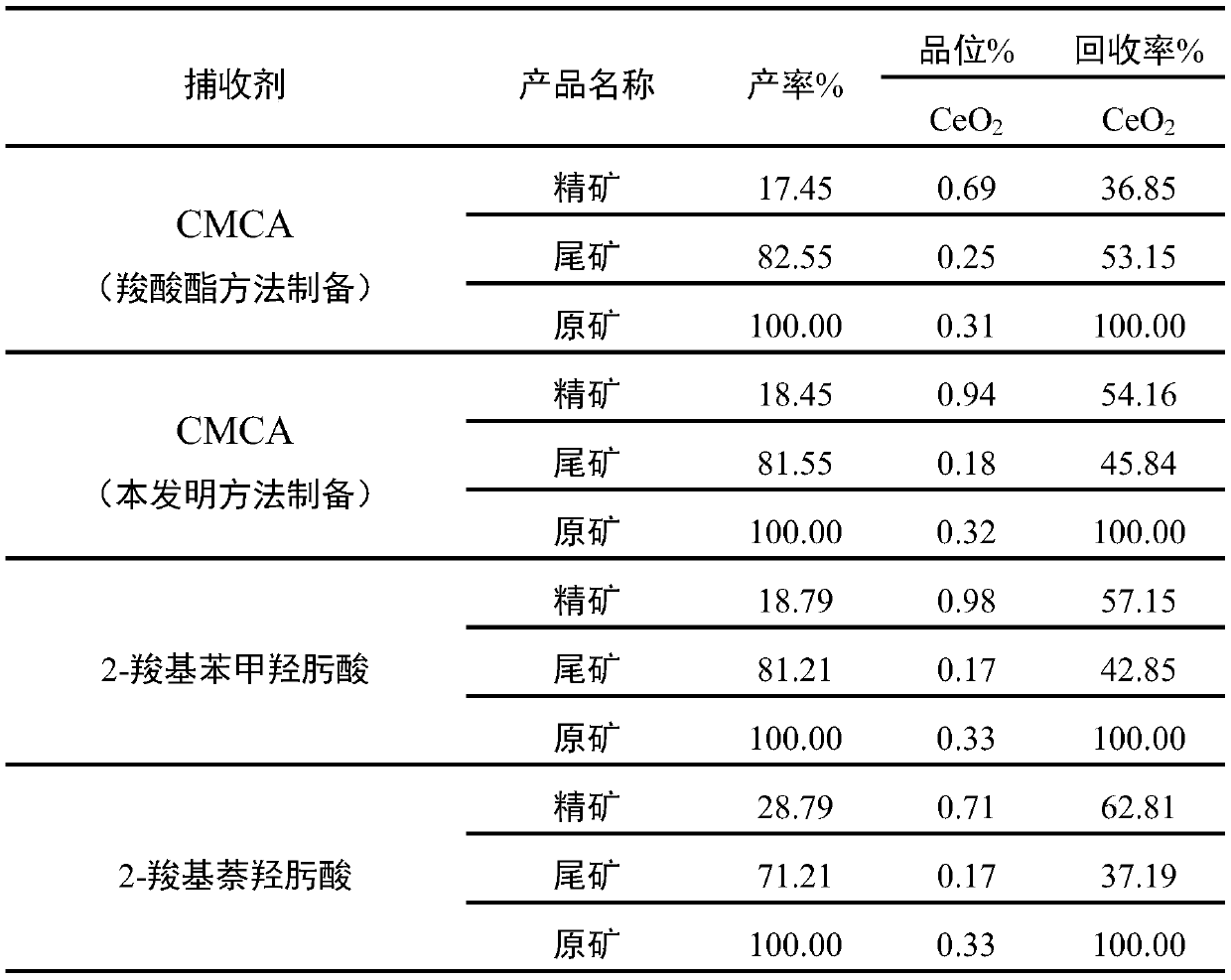
Image
Examples
Embodiment 1
[0029] The present embodiment provides a kind of preparation method of 2-carboxynaphthalene hydroxamic acid, comprises the following steps:
[0030] Put the reaction bottle into the ice-water mixture for cooling, add hydroxylamine hydrochloride and naphthalic anhydride with a molar ratio of 1.3:1.0 therein, and add water to dissolve it; under stirring, add 40 wt% NaOH aqueous solution dropwise to control the rate of addition Keep the reaction temperature below 30°C, the molar ratio of naphthalic anhydride to NaOH is 1:1.6, continue to stir for 5 hours after the NaOH aqueous solution is added dropwise, to ensure sufficient reaction; then add 30wt% dilute sulfuric acid dropwise for acidification, Acidify to pH 6, and finally filter and separate the reaction solution to obtain 2-carboxynaphthalene hydroxamic acid as a light yellow solid with a product yield of 91.5% and a purity of 70.5%.
Embodiment 2
[0032] The present embodiment provides a kind of preparation method of 2-carboxybenzohydroxamic acid, comprises the following steps:
[0033] Put the reaction bottle into the ice-water mixture to cool, add hydroxylamine hydrochloride and phthalic anhydride with a molar ratio of 1.25:1.0, and add water to dissolve it; under stirring, add 40 wt% NaOH aqueous solution dropwise to control the drop Acceleration keeps the reaction temperature below 30°C, the molar ratio of phthalic anhydride to NaOH is 1:1.5, and the NaOH aqueous solution is added dropwise and then stirred for 2 hours to ensure sufficient reaction; then 30wt% dilute sulfuric acid is added dropwise Acidification until the pH is 5, and finally the reaction solution is separated by filtration to obtain 2-carboxyphenylhydroxamic acid as a white solid with a product yield of 92.8% and a purity of 70.2%.
Embodiment 3
[0035] The present embodiment provides a kind of preparation method of 3-carboxy-octenyl hydroxamic acid (CMCA), comprises the following steps:
[0036] The reaction bottle is put into the ice-water mixture for cooling, and the hydroxylamine hydrochloride and pentylmaleic anhydride that the molar ratio is 1.35:1.0 are added thereto, and water is added to make it dissolve; Acceleration keeps the reaction temperature below 30°C, and the molar ratio of acid anhydride to NaOH is 1:1.65. After the NaOH aqueous solution is added dropwise, continue to stir for 3 hours to ensure sufficient reaction; then add 30wt% dilute sulfuric acid dropwise to The pH is 5, and finally the reaction solution is separated by filtration to obtain CMCA as a pasty product with a yield of 95.8% and a purity of 75.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com