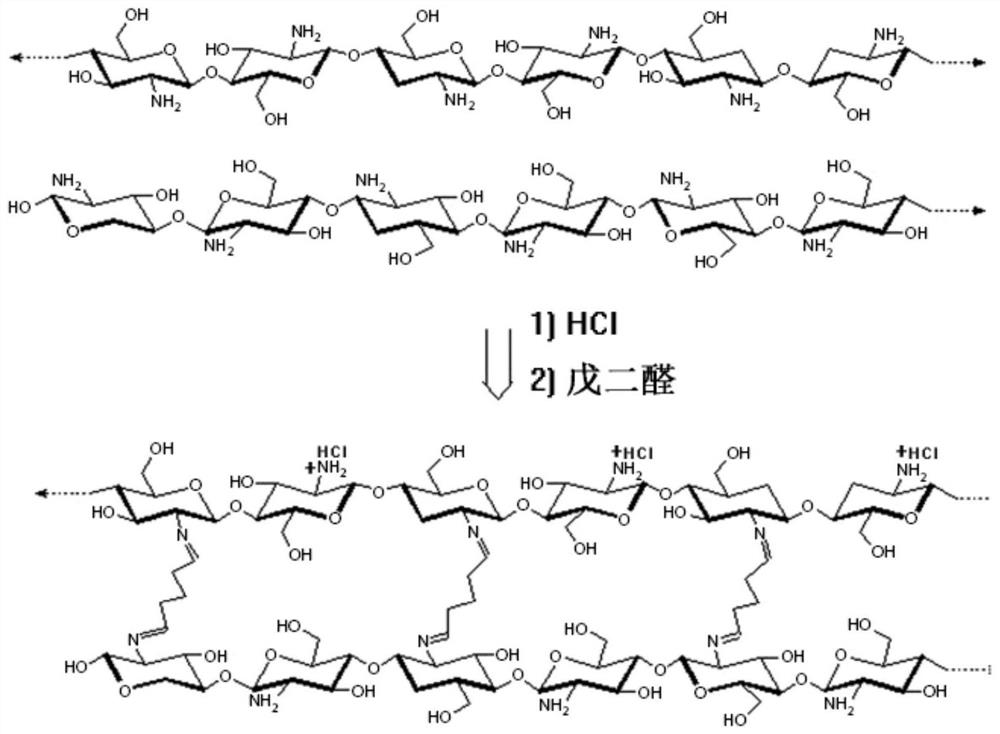
A method for preparing carbon aerogels from hydrogel precursor materials through a hydrothermal process
A precursor material, carbon aerogel technology, applied in aerogel preparation, colloid chemistry, colloid chemistry and other directions, can solve the problems of low overall yield, many preparation process steps, no application value, etc., to achieve overall yield High, easy structure control effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Chitosan 1.6g, 36wt% concentrated hydrochloric acid 1.0g, water-soluble carbohydrate 20g and deionized water 77.4g form a uniform solution; then add 1.2g Tween-60 and 5.0g liquid paraffin, stir to form a uniformly dispersed system; dropwise 2.2g glutaraldehyde aqueous solution (50wt%), after stirring evenly, leave standstill to form hydrogel; The hydrogel is placed in the stainless steel reaction kettle that has polytetrafluoroethylene liner, left standstill reaction 4.0 hours at 180 ℃; Reaction After finishing, the primary carbonized product is cleaned to a neutral pH value by deionized water, and then by ethanol-n-butanol (V / V:2 / 1, 45g), n-butanol-n-hexane (V / V:3 / 1 , 50g) The system is subjected to solvent replacement in sequence, and the replaced primary carbonized product is vacuum-dried at room temperature until the quality remains unchanged; the dried primary carbonized product is further heat-treated at 800°C, and the heating rate of the process is 3.0°C / min. The...
Embodiment 2
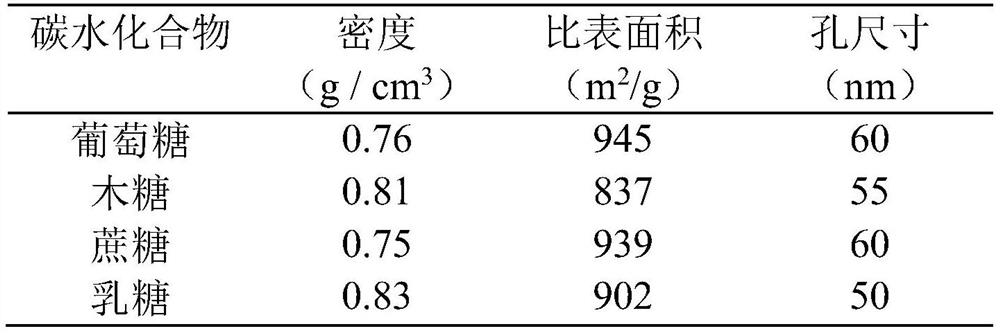
[0059] Chitosan, 36wt% concentrated hydrochloric acid, glucose and deionized water form a uniform solution; then add Tween-60 and liquid paraffin, stir to form a uniformly dispersed system; dropwise add glutaraldehyde aqueous solution (50wt%) (concrete hydrogel composition The conditions are shown in Table 2), stirred evenly and then left to stand to form a hydrogel; the hydrogel was placed in a stainless steel reactor with a polytetrafluoroethylene lining, and was left to react at 180°C for 4.0 hours; after the reaction, the primary The carbonized product is cleaned by deionized water until the pH value is neutral, then by ethanol-n-butanol (V / V:2 / 1, 45g), n-butanol-n-hexane (V / V:3 / 1, 50g) The system was subjected to solvent replacement in sequence, and the replaced primary carbonized products were vacuum-dried at room temperature until the quality remained unchanged; the dried primary carbonized products were further heat-treated at 800 °C, the heating rate of the process was...
Embodiment 3
[0066] Chitosan 1.6g, 36wt% concentrated hydrochloric acid 1.0g, glucose 20g and deionized water 77.4g form a uniform solution; then add 1.2g Tween-60 and 5.0g liquid paraffin, stir to form a dispersed uniform system; Dialdehyde aqueous solution (50wt%), stirred evenly and left to stand to form a hydrogel; the hydrogel was placed in a stainless steel reaction kettle with a polytetrafluoroethylene lining, and was left to react at 180 ° C for 4.0 hours; after the reaction, the primary The carbonized product is cleaned by deionized water until the pH value is neutral, and then the solvent replacement is carried out in sequence by ethanol-n-butanol and n-butanol-n-hexane system (solvent replacement conditions are shown in Table 4), and the replaced primary carbonized product Vacuum drying at room temperature until the quality remains unchanged; the dried primary carbonized product is further heat-treated at 800°C, the heating rate of the process is 3.0°C / min, the nitrogen flow rate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com