High-efficiency catalyst and method for catalyzing formic acid dehydrogenation and reduction reaction
A catalyst, formic acid technology, applied in catalytic reactions, chemical instruments and methods, catalysts for physical/chemical processes, etc., can solve the problem of low atomic economy, and achieve the effects of excellent water solubility, high efficiency, and low catalyst dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
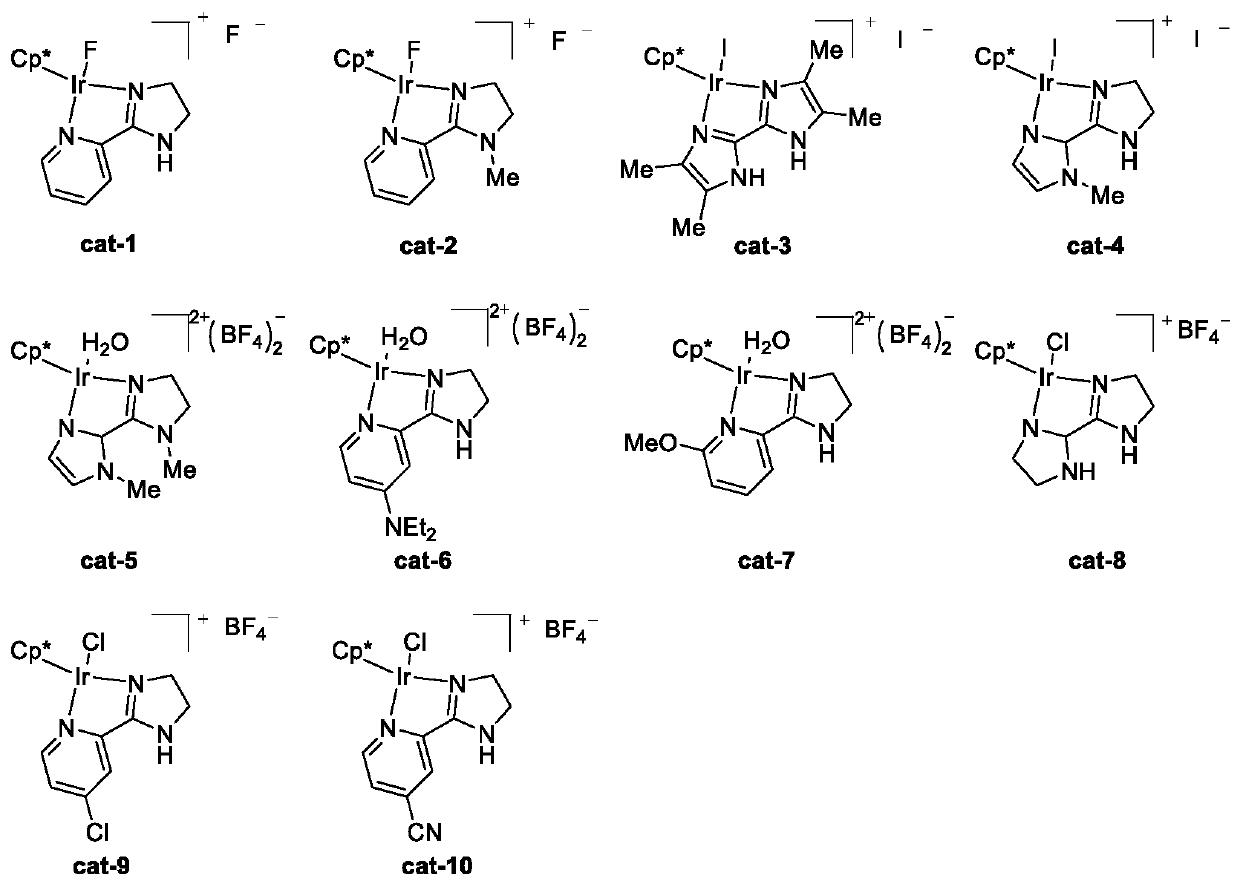
[0050] Catalyst cat-1 (structure see figure 1 ) preparation:
[0051] To a 100 mL round bottom flask was added the ligand 2-(4,5-dihydro-1-imidazole)pyridine (440 mg, 3 mmol), [Cp*IrCl 2 ] 2 (1.2g, 1.5mmol) and 30mL of dichloromethane, stirred until all the solids were dissolved, stirred at room temperature for 12h, then added silver tetrafluoroborate (585mg, 3mmol) to the flask, stirred for 30 minutes and then added sodium fluoride ( 126mg, 3mmol) continued to stir for 3h, then removed the solvent on a rotary evaporator, and the obtained solid was washed 3 times with ethyl acetate to obtain a tan solid, which was the catalyst cat-1 (1.2g, 2.3mmol), the yield 77%.
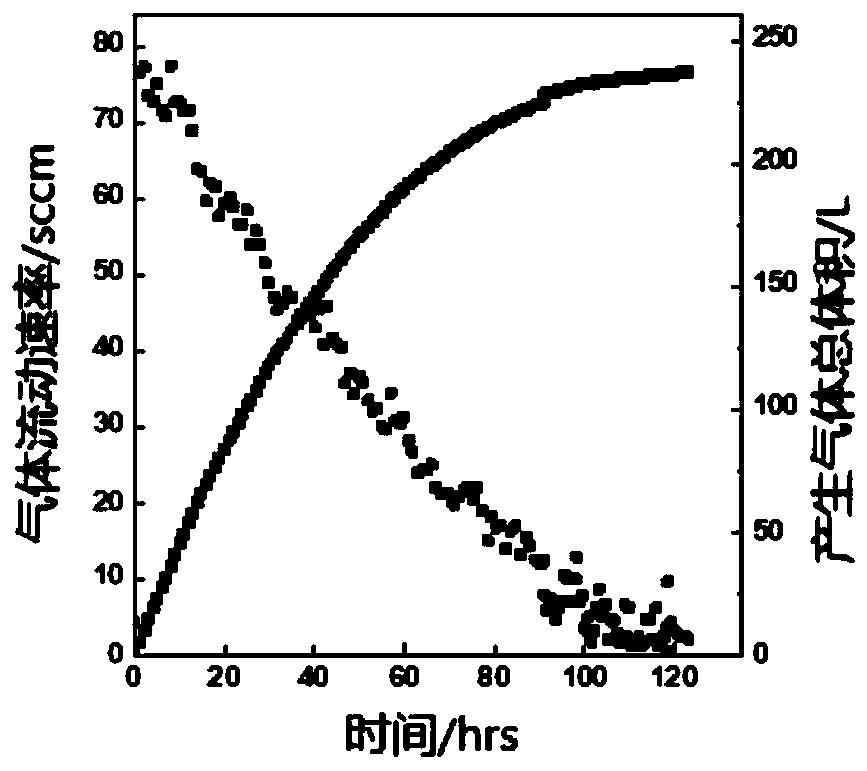
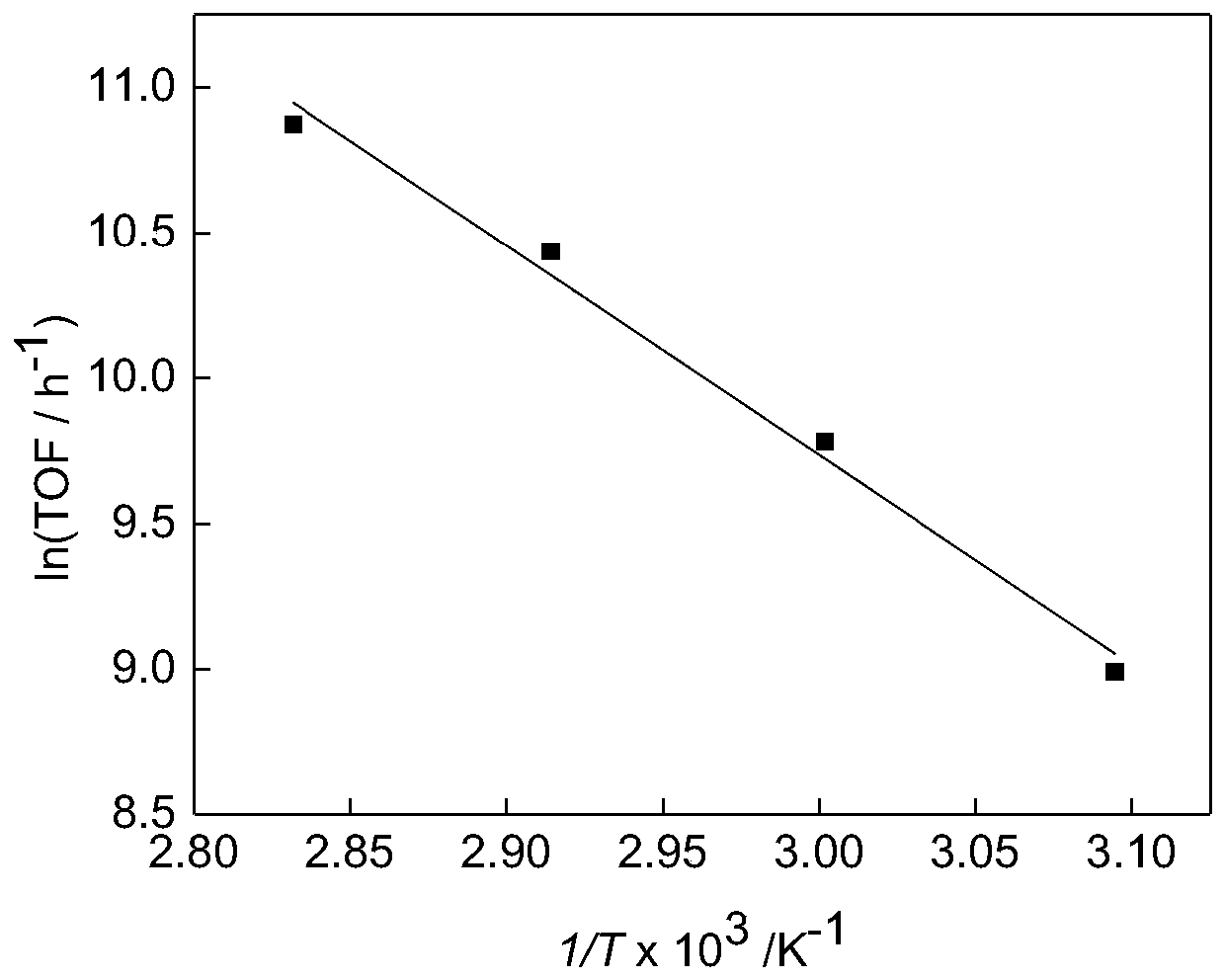
[0052] The catalyst cat-1 (5.6 mg, 10 μmol) was weighed and dissolved in 1 mL of deionized water, mixed to form a homogeneous solution, and then 0.1 mL of the mixed solution was taken and diluted to 10 mL with deionized water and placed in a 50 mL round bottom flask. Put the round-bottomed flask containing the ...
Embodiment 2
[0056] Others are the same as embodiment 1, but the catalyst used is changed into cat-2 (structure sees figure 1 , the preparation method is the same as cat-1, except that the ligand is changed to 2-(1-methyl-4,5-dihydro-1-imidazole)pyridine), and the results are shown in Table 1.
Embodiment 3
[0058] Others are the same as embodiment 1, but the catalyst used is changed into cat-3 (structure sees figure 1 , the preparation method is the same as cat-1, except that the ligand is changed to 4,4',5,5'-tetramethyl-1H, 1'H-2,2'-diimidazole), and the results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com