Preparation method of methylene blue hapten and methylene blue immunogen
A methylene blue and hapten technology, applied in chemical instruments and methods, animal/human proteins, albumin peptides, etc., can solve the problems of short exposed groups and difficulty in producing polyclonal antibodies, and achieve the effect of easy availability of raw materials and competitive inhibition Excellent, easy-to-operate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
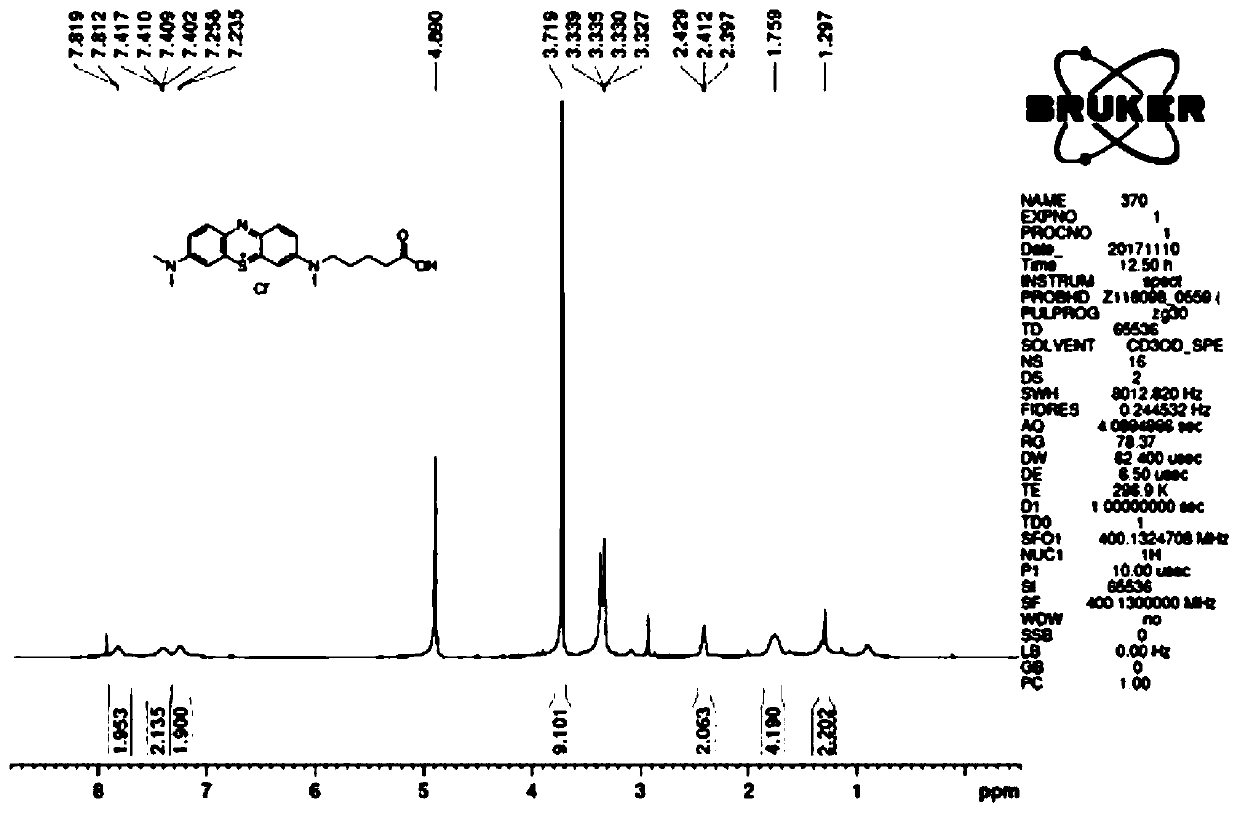
[0035] Example 1: Preparation of methylene blue hapten
[0036] 1, Synthesis of 2-amino-5-dimethylaminophenylthiosulfuric acid (intermediate 1)
[0037]
[0038] Add aluminum sulfate 18 hydrate, sodium thiosulfate, zinc chloride and N,N-dimethyl-p-phenylenediamine in a molar ratio of 1:2:1:1 until the number of moles is equal to the number of moles of the above-mentioned reaction raw materials 3 to 4 times the total amount of water, the reaction temperature is 0°C, and the stirring time is 5 to 10 minutes.
[0039] Then, according to the molar ratio of potassium dichromate to aluminum sulfate 18 hydrate of 1:3.5-4.0, drop 0.5-1.0mol / L potassium dichromate solution, react at 0°C for 2 hours, and obtain the product; slowly rise to room temperature , suction filtration, the filter cake was successively washed with an equal volume of the reaction solution with water and half the volume of acetone, drained by an oil pump, and beaten with methyl tert-butyl ether with an equal vo...
Embodiment 2
[0048] Embodiment 2, the preparation of methylene blue immunogen
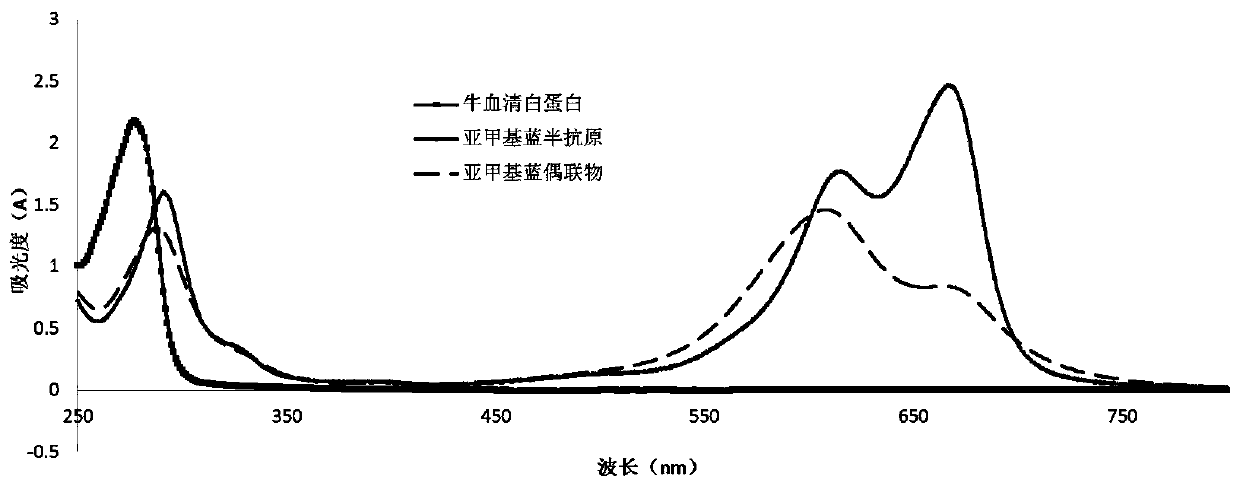
[0049] The methylene blue hapten prepared in Example 1, dicyclohexylcarbodiimide (DCC) and N-hydroxysuccinimide (NHS) were put into a beaker according to a molar ratio of 1:1:1, and Add dimethylformamide (DMF) solution to the beaker, dissolve it completely, and react under magnetic stirring for 8 hours at room temperature, then let it stand overnight at 4°C; after the reaction, centrifuge at 4°C, 4000r.p.m 10min, take the supernatant. Add the above supernatant to 5 mL of BSA solution with a concentration of 10-20 mg / mL, and react in an ice-water bath for 6 h. After the reaction, the solution was transferred to the treated dialysis bag, and dialyzed at 4° C. for 3 days with phosphate buffer solution as the dialysate, and the dialysate was changed 3-4 times a day. After dialysis, at 4°C,
[0050] Centrifuge at 4000r.p.m for 10min, and take the supernatant, which is the methylene blue immunogen. Through UV sca...
Embodiment 3
[0051] Embodiment 3, the result verification of methylene blue immunogenicity
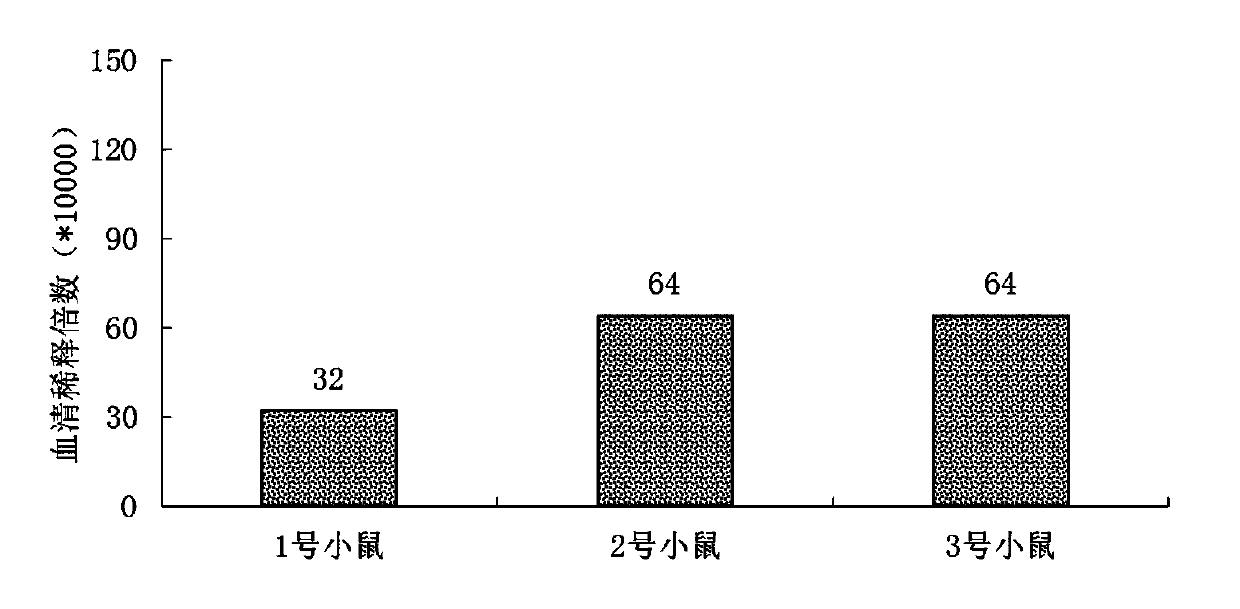
[0052] Dilute the methylene blue immunogen with phosphate buffer solution to obtain a 2 mg / mL immunogen solution, add 100 μL of the immunogen solution to 100 μL of Freund’s complete adjuvant, mix well, take three 4-week-old Balb / c mice, and use Abdominal injection for immunization; two weeks after immunization, take 100 μL of immunogen solution and add 100 μL of Freund’s incomplete adjuvant, mix well, and then use abdominal injection for immunization; one week later, take 100 μL of immunogen solution for tail vein booster immunization Ten days later, 50 μL of the immunogen solution was injected into the tail vein for booster immunization. On the third day of the last immunization, blood was collected from the orbit, bathed in water at 37°C for 1 hour, the supernatant was taken, and the serum titer and sensitivity were determined by ELISA method.
[0053] according to image 3 , the immune antibod...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com